Abstract
A phase II trial investigating the anti-tumour effects of recombinant human interleukin 6 (rhIL-6) in patients with metastatic renal cell cancer was carried out. RhIL-6 (150 microgram) was administered as a daily subcutaneous injection for 42 consecutive days on an outpatient basis. Forty-nine patients were studied, 12 with and 37 without previous immunotherapy. Forty patients were evaluable for response. A partial remission was noted in two patients, stable disease in 17 and progressive disease in 21. Toxicity was moderate and reversible and consisted mainly of fever, flu-like symptoms, nausea, weight loss and hepatotoxicity. Anaemia, leucocytosis and thrombocytosis and induction of acute phase protein synthesis were noted in most patients. In 15% of the patients anti-IL-6 antibodies developed, and were neutralising in only one patient. Baseline plasma IL-6 concentrations did not correlate with tumour behaviour before or after rhIL-6 treatment. In conclusion, rhIL-6 can be safely administered on an outpatient basis for prolonged period of time and has moderate, reversible toxicity. Its administration induces IL-6-antibody production in only a minority of patients. Antitmour effects of rhIL-6 in metastatic renal cancer are limited.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blay J. Y., Negrier S., Combaret V., Attali S., Goillot E., Merrouche Y., Mercatello A., Ravault A., Tourani J. M., Moskovtchenko J. F. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992 Jun 15;52(12):3317–3322. [PubMed] [Google Scholar]
- Chen L., Mory Y., Zilberstein A., Revel M. Growth inhibition of human breast carcinoma and leukemia/lymphoma cell lines by recombinant interferon-beta 2. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8037–8041. doi: 10.1073/pnas.85.21.8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
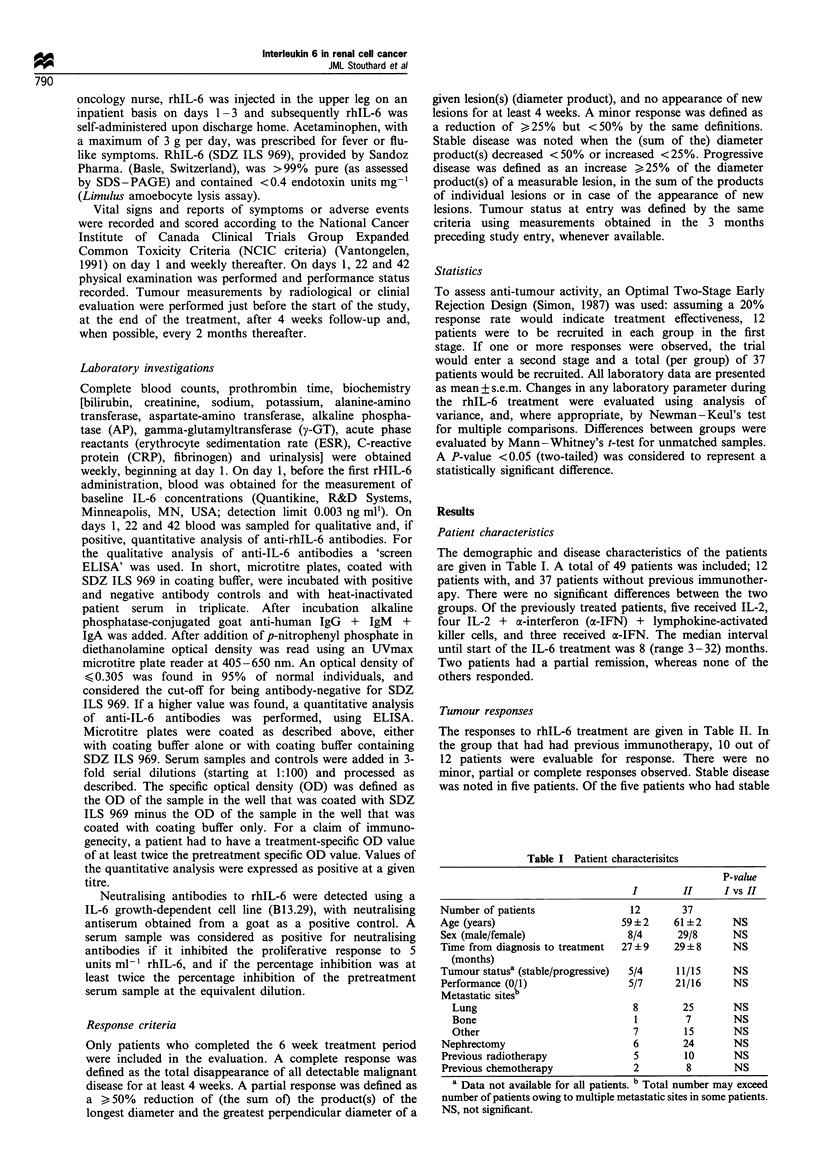
- Gauldie J., Richards C., Baumann H. IL6 and the acute phase reaction. Res Immunol. 1992 Sep;143(7):755–759. doi: 10.1016/0923-2494(92)80018-g. [DOI] [PubMed] [Google Scholar]
- Gogusev J., Augusti M., Chrétien Y., Droz D. Interleukin-6 and TNF alpha production in human renal cell carcinoma. Kidney Int. 1993 Sep;44(3):585–592. doi: 10.1038/ki.1993.285. [DOI] [PubMed] [Google Scholar]
- Gruss H. J., Brach M. A., Mertelsmann R. H., Herrmann F. Interferon-gamma interrupts autocrine growth mediated by endogenous interleukin-6 in renal-cell carcinoma. Int J Cancer. 1991 Nov 11;49(5):770–773. doi: 10.1002/ijc.2910490523. [DOI] [PubMed] [Google Scholar]
- Houssiau F., Van Snick J. IL6 and the T-cell response. Res Immunol. 1992 Sep;143(7):740–743. doi: 10.1016/0923-2494(92)80014-c. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Asano S. IL6 and thrombocytopoiesis. Res Immunol. 1992 Sep;143(7):752–754. doi: 10.1016/0923-2494(92)80017-f. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
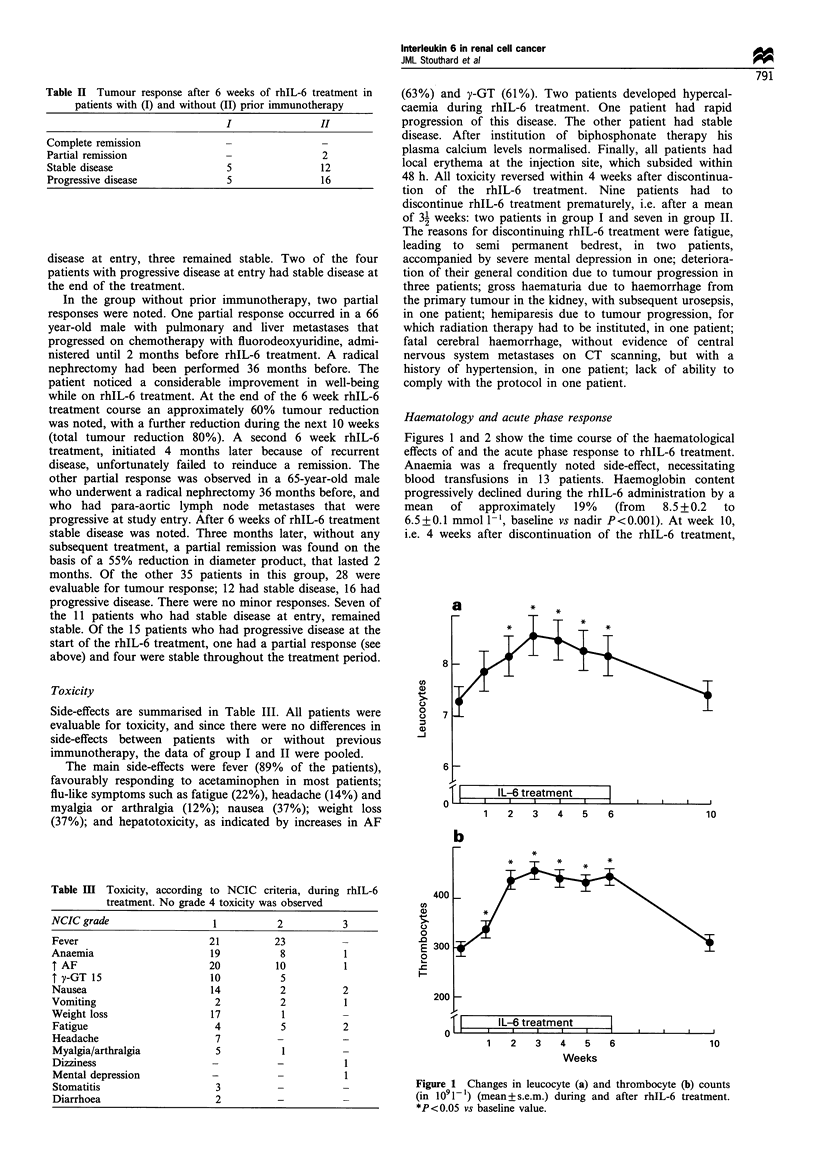
- Koo A. S., Armstrong C., Bochner B., Shimabukuro T., Tso C. L., deKernion J. B., Belldegrum A. Interleukin-6 and renal cell cancer: production, regulation, and growth effects. Cancer Immunol Immunother. 1992;35(2):97–105. doi: 10.1007/BF01741856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J. M., Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989 Dec;61(6):588–602. [PubMed] [Google Scholar]
- Miki S., Iwano M., Miki Y., Yamamoto M., Tang B., Yokokawa K., Sonoda T., Hirano T., Kishimoto T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989 Jul 3;250(2):607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
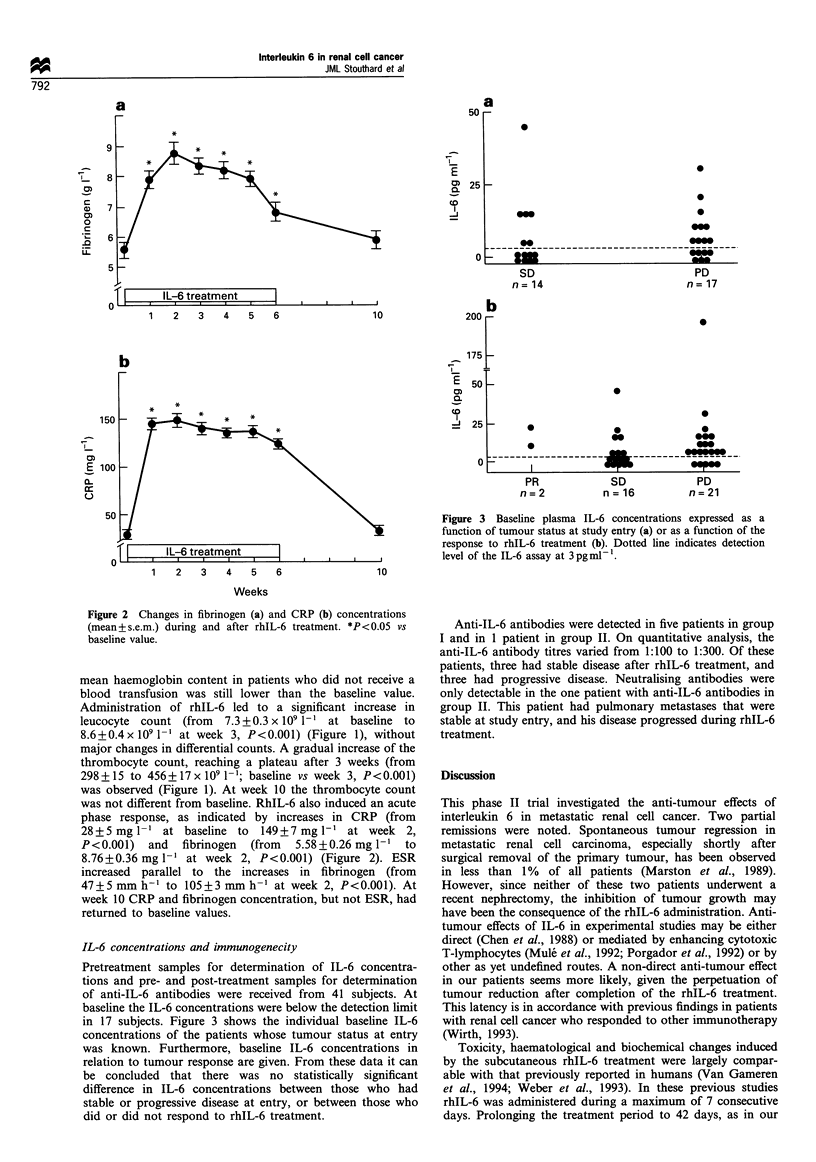
- Mulé J. J., Custer M. C., Travis W. D., Rosenberg S. A. Cellular mechanisms of the antitumor activity of recombinant IL-6 in mice. J Immunol. 1992 Apr 15;148(8):2622–2629. [PubMed] [Google Scholar]
- Mulé J. J., McIntosh J. K., Jablons D. M., Rosenberg S. A. Antitumor activity of recombinant interleukin 6 in mice. J Exp Med. 1990 Mar 1;171(3):629–636. doi: 10.1084/jem.171.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porgador A., Tzehoval E., Katz A., Vadai E., Revel M., Feldman M., Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumor cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992 Jul 1;52(13):3679–3686. [PubMed] [Google Scholar]
- Ravoet C., DeGrève J., Vandewoude K., Kerger J., Sculier J. P., Lacor P., Stryckmans P., Piccart M. Tumour stimulating effects of recombinant human interleukin-6. Lancet. 1994 Dec 3;344(8936):1576–1577. doi: 10.1016/s0140-6736(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Revel M. Growth regulatory functions of IL6 and antitumour effects. Res Immunol. 1992 Sep;143(7):769–773. doi: 10.1016/0923-2494(92)80021-c. [DOI] [PubMed] [Google Scholar]
- Simon R. How large should a phase II trial of a new drug be? Cancer Treat Rep. 1987 Nov;71(11):1079–1085. [PubMed] [Google Scholar]
- Stadler W. M., Richards J. M., Vogelzang N. J. Serum interleukin-6 levels in metastatic renal cell cancer: correlation with survival but not an independent prognostic indicator. J Natl Cancer Inst. 1992 Dec 2;84(23):1835–1836. doi: 10.1093/jnci/84.23.1835. [DOI] [PubMed] [Google Scholar]
- Stouthard J. M., Romijn J. A., Van der Poll T., Endert E., Klein S., Bakker P. J., Veenhof C. H., Sauerwein H. P. Endocrinologic and metabolic effects of interleukin-6 in humans. Am J Physiol. 1995 May;268(5 Pt 1):E813–E819. doi: 10.1152/ajpendo.1995.268.5.E813. [DOI] [PubMed] [Google Scholar]
- Stouthard J. M., van der Poll T., Endert E., Bakker P. J., Veenhof C. H., Sauerwein H. P., Romijn J. A. Effects of acute and chronic interleukin-6 administration on thyroid hormone metabolism in humans. J Clin Endocrinol Metab. 1994 Nov;79(5):1342–1346. doi: 10.1210/jcem.79.5.7962327. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Brouckaert P., Fiers W. Induction of tolerance allows separation of lethal and antitumor activities of tumor necrosis factor in mice. Cancer Res. 1991 May 1;51(9):2366–2372. [PubMed] [Google Scholar]
- Tsukamoto T., Kumamoto Y., Miyao N., Masumori N., Takahashi A., Yanase M. Interleukin-6 in renal cell carcinoma. J Urol. 1992 Dec;148(6):1778–1782. doi: 10.1016/s0022-5347(17)37026-x. [DOI] [PubMed] [Google Scholar]
- Weber J., Yang J. C., Topalian S. L., Parkinson D. R., Schwartzentruber D. S., Ettinghausen S. E., Gunn H., Mixon A., Kim H., Cole D. Phase I trial of subcutaneous interleukin-6 in patients with advanced malignancies. J Clin Oncol. 1993 Mar;11(3):499–506. doi: 10.1200/JCO.1993.11.3.499. [DOI] [PubMed] [Google Scholar]
- Wirth M. P. Immunotherapy for metastatic renal cell carcinoma. Urol Clin North Am. 1993 May;20(2):283–295. [PubMed] [Google Scholar]
- Yagoda A., Petrylak D., Thompson S. Cytotoxic chemotherapy for advanced renal cell carcinoma. Urol Clin North Am. 1993 May;20(2):303–321. [PubMed] [Google Scholar]
- van Gameren M. M., Willemse P. H., Mulder N. H., Limburg P. C., Groen H. J., Vellenga E., de Vries E. G. Effects of recombinant human interleukin-6 in cancer patients: a phase I-II study. Blood. 1994 Sep 1;84(5):1434–1441. [PubMed] [Google Scholar]


