Abstract
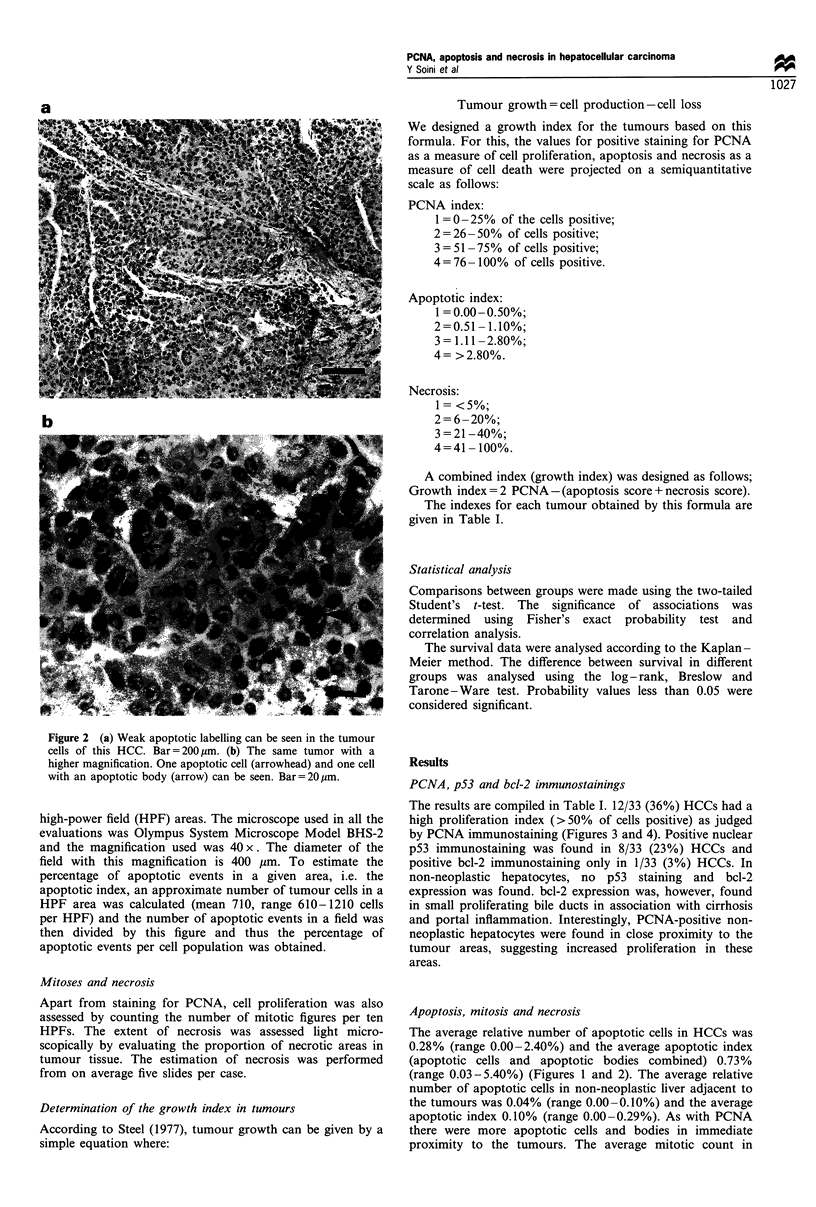
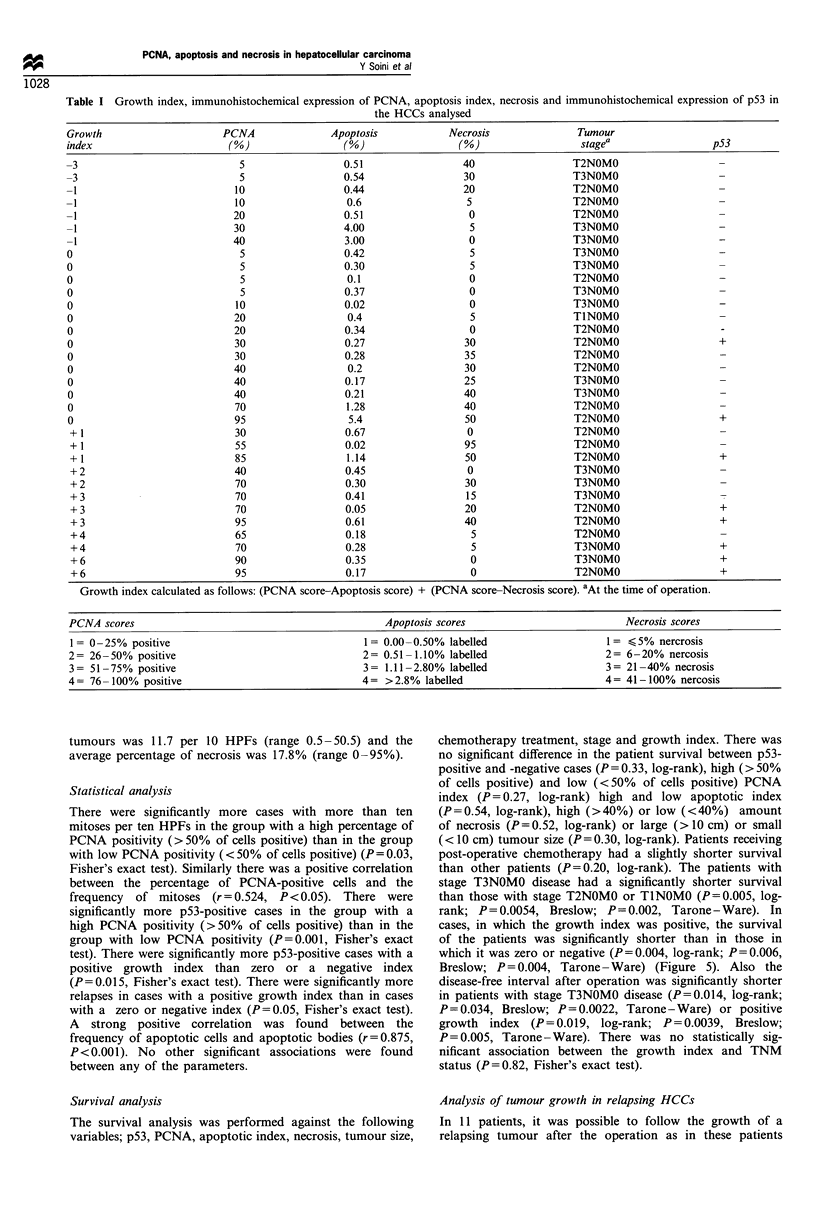
In this study we investigated tumour growth in relation to the immunohistochemical expression of p53 and bcl-2 and to patient survival data in 33 operated hepatocellular carcinomas (HCCs). In order to estimate the growth, a growth index, based on the degree of cell proliferation, apoptosis and necrosis, was calculated for each tumour. Cell proliferation was determined immunohistochemically by the number of proliferating cell nuclear antigen (PCNA)-positive cells in tumours, the extent of apoptosis was determined by counting the number of cells labelled by the in situ 3'-end labelling technique and tumour necrosis was estimated as the percentage of necrotic areas in haematoxylin--eosin-stained tissue sections. In our analysis we found that the survival of patients with HCCs showing a high growth index (i.e. tumours showing a high proliferation and simultaneously a low degree of apoptosis and necrosis) was significantly shorter than with other patients (P = 0.004, log-rank test). When analysed separately, cell proliferation, apoptosis or necrosis did not show any significant association with survival. p53 positivity was found in 8/33 (24%) of tumours. There were significantly more p53-positive cases in tumours with a high growth index (P = 0.01, Fisher's exact test) suggesting that dysfunction of the p53 gene may affect tumour growth. p53-positive cases did not, however, have a significantly shorter survival time than p53-negative cases (P = 0.3, log-rank test). bcl-2 positivity was found in only 1/33 (3%) of the HCCs. Thus bcl-2 overexpression does not seem to play an important role in hepatocellular carcinogenesis. In summary, our results suggest that in HCCs a compound score based on the evaluation of the degree of cell proliferation, apoptosis and necrosis is a biologically more relevant prognostic indicator than any of its composite parameters alone.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charlotte F., L'Herminé A., Martin N., Geleyn Y., Nollet M., Gaulard P., Zafrani E. S. Immunohistochemical detection of bcl-2 protein in normal and pathological human liver. Am J Pathol. 1994 Mar;144(3):460–465. [PMC free article] [PubMed] [Google Scholar]
- Collier J. D., Carpenter M., Burt A. D., Bassendine M. F. Expression of mutant p53 protein in hepatocellular carcinoma. Gut. 1994 Jan;35(1):98–100. doi: 10.1136/gut.35.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMONDSON H. A., STEINER P. E. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954 May;7(3):462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Haldar S., Negrini M., Monne M., Sabbioni S., Croce C. M. Down-regulation of bcl-2 by p53 in breast cancer cells. Cancer Res. 1994 Apr 15;54(8):2095–2097. [PubMed] [Google Scholar]
- Laurent-Puig P., Flejou J. F., Fabre M., Bedossa P., Belghiti J., Gayral F., Franco D. Overexpression of p53: a rare event in a large series of white patients with hepatocellular carcinoma. Hepatology. 1992 Nov;16(5):1171–1175. [PubMed] [Google Scholar]
- Miyashita T., Krajewski S., Krajewska M., Wang H. G., Lin H. K., Liebermann D. A., Hoffman B., Reed J. C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994 Jun;9(6):1799–1805. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995 Jan 27;80(2):293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Soini Y., Päkkö P., Nuorva K., Kamel D., Lane D. P., Vähäkangas K. Comparative analysis of p53 protein immunoreactivity in prostatic, lung and breast carcinomas. Virchows Arch A Pathol Anat Histopathol. 1992;421(3):223–228. doi: 10.1007/BF01611179. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Törmänen U., Eerola A. K., Rainio P., Vähäkangas K., Soini Y., Sormunen R., Bloigu R., Lehto V. P., Päkkö P. Enhanced apoptosis predicts shortened survival in non-small cell lung carcinoma. Cancer Res. 1995 Dec 1;55(23):5595–5602. [PubMed] [Google Scholar]
- Volkmann M., Hofmann W. J., Müller M., Räth U., Otto G., Zentgraf H., Galle P. R. p53 overexpression is frequent in European hepatocellular carcinoma and largely independent of the codon 249 hot spot mutation. Oncogene. 1994 Jan;9(1):195–204. [PubMed] [Google Scholar]
- Wands J. R., Blum H. E. Primary hepatocellular carcinoma. N Engl J Med. 1991 Sep 5;325(10):729–731. doi: 10.1056/NEJM199109053251010. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]






