Abstract
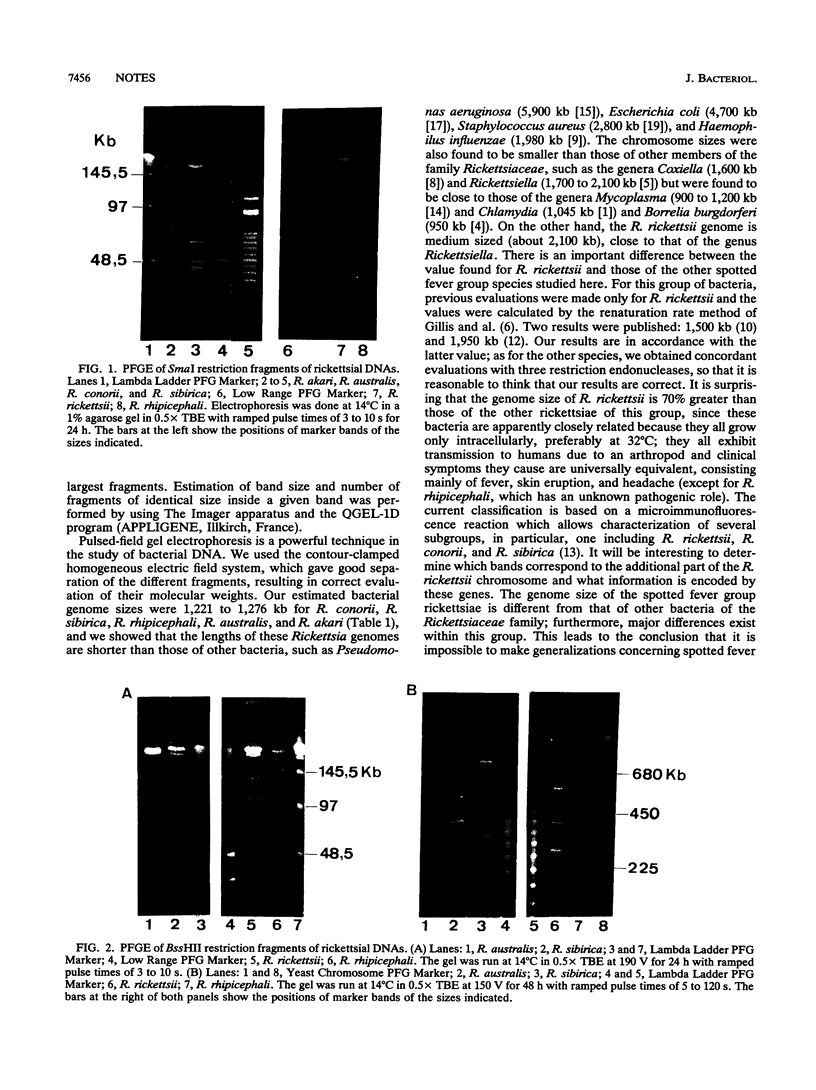
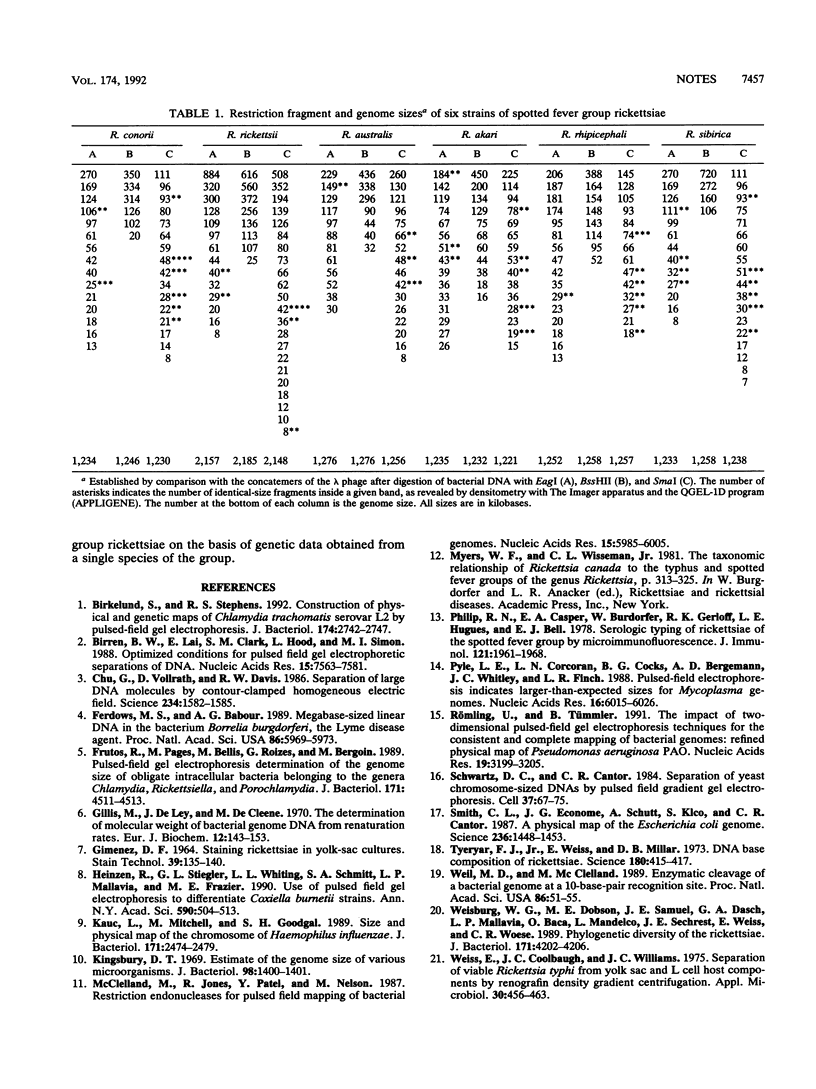
The chromosome lengths of six spotted fever group Rickettsia species (Rickettsia rickettsii, R. conorii, R. rhipicephali, R. sibirica, R. australis, and R. akari) were estimated by pulsed-field gel electrophoresis. The genome size of R. rickettsii was about 2,100 kb, but the chromosome lengths of the five other species were, surprisingly, much lower and ranged between 1,200 and 1,300 kb.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkelund S., Stephens R. S. Construction of physical and genetic maps of Chlamydia trachomatis serovar L2 by pulsed-field gel electrophoresis. J Bacteriol. 1992 May;174(9):2742–2747. doi: 10.1128/jb.174.9.2742-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren B. W., Lai E., Clark S. M., Hood L., Simon M. I. Optimized conditions for pulsed field gel electrophoretic separations of DNA. Nucleic Acids Res. 1988 Aug 11;16(15):7563–7582. doi: 10.1093/nar/16.15.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frutos R., Pages M., Bellis M., Roizes G., Bergoin M. Pulsed-field gel electrophoresis determination of the genome size of obligate intracellular bacteria belonging to the genera Chlamydia, Rickettsiella, and Porochlamydia. J Bacteriol. 1989 Aug;171(8):4511–4513. doi: 10.1128/jb.171.8.4511-4513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Heinzen R., Stiegler G. L., Whiting L. L., Schmitt S. A., Mallavia L. P., Frazier M. E. Use of pulsed field gel electrophoresis to differentiate Coxiella burnetii strains. Ann N Y Acad Sci. 1990;590:504–513. doi: 10.1111/j.1749-6632.1990.tb42260.x. [DOI] [PubMed] [Google Scholar]
- Kauc L., Mitchell M., Goodgal S. H. Size and physical map of the chromosome of Haemophilus influenzae. J Bacteriol. 1989 May;171(5):2474–2479. doi: 10.1128/jb.171.5.2474-2479.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- Pyle L. E., Corcoran L. N., Cocks B. G., Bergemann A. D., Whitley J. C., Finch L. R. Pulsed-field electrophoresis indicates larger-than-expected sizes for mycoplasma genomes. Nucleic Acids Res. 1988 Jul 11;16(13):6015–6025. doi: 10.1093/nar/16.13.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Weiss E., Millar D. B., Bozeman F. M., Ormsbee R. A. DNA base composition of rickettsiae. Science. 1973 Apr 27;180(4084):415–417. doi: 10.1126/science.180.4084.415. [DOI] [PubMed] [Google Scholar]
- Weil M. D., McClelland M. Enzymatic cleavage of a bacterial genome at a 10-base-pair recognition site. Proc Natl Acad Sci U S A. 1989 Jan;86(1):51–55. doi: 10.1073/pnas.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Dobson M. E., Samuel J. E., Dasch G. A., Mallavia L. P., Baca O., Mandelco L., Sechrest J. E., Weiss E., Woese C. R. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989 Aug;171(8):4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]