Abstract
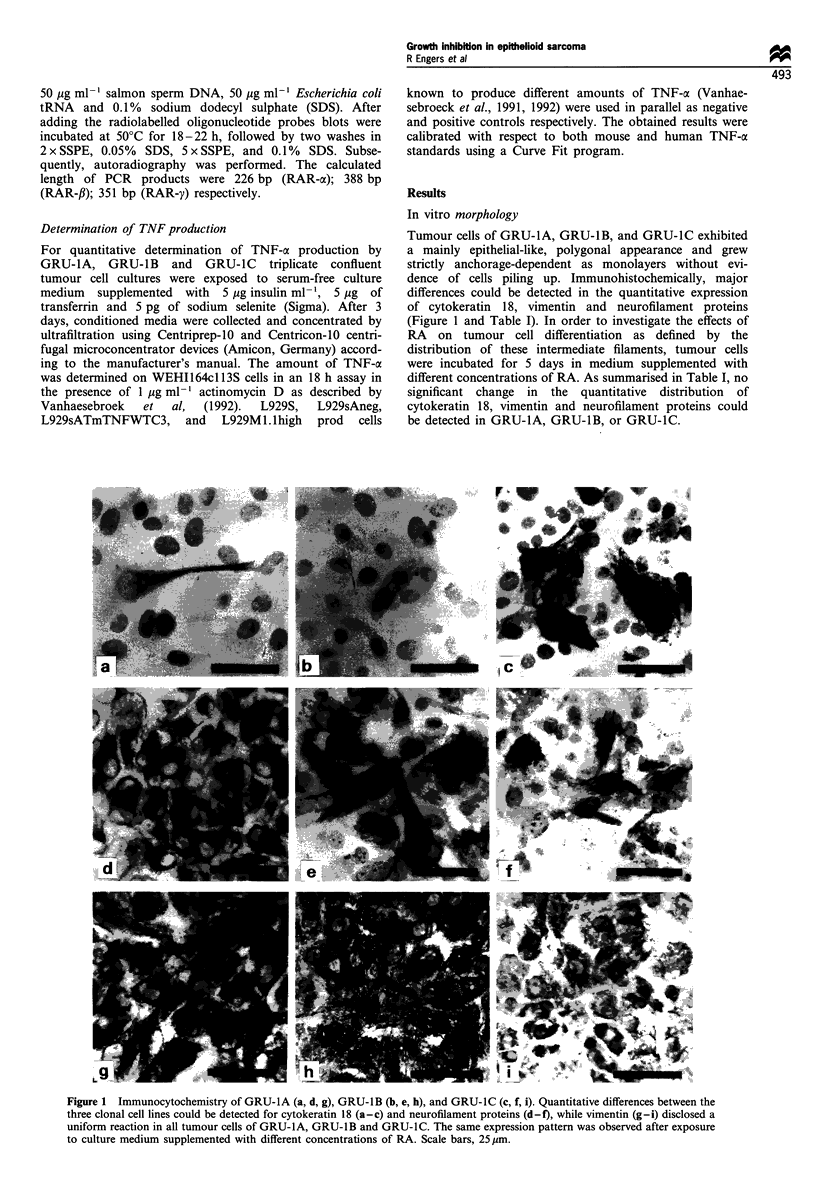
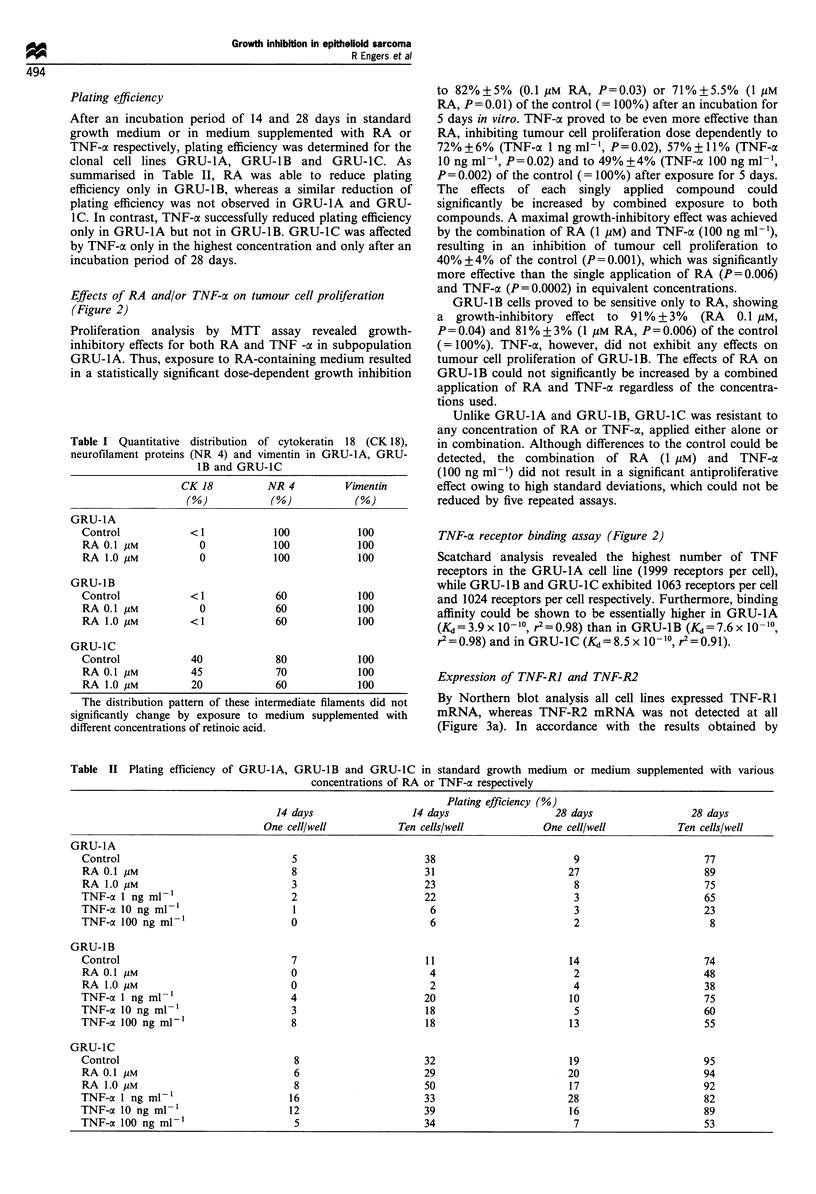
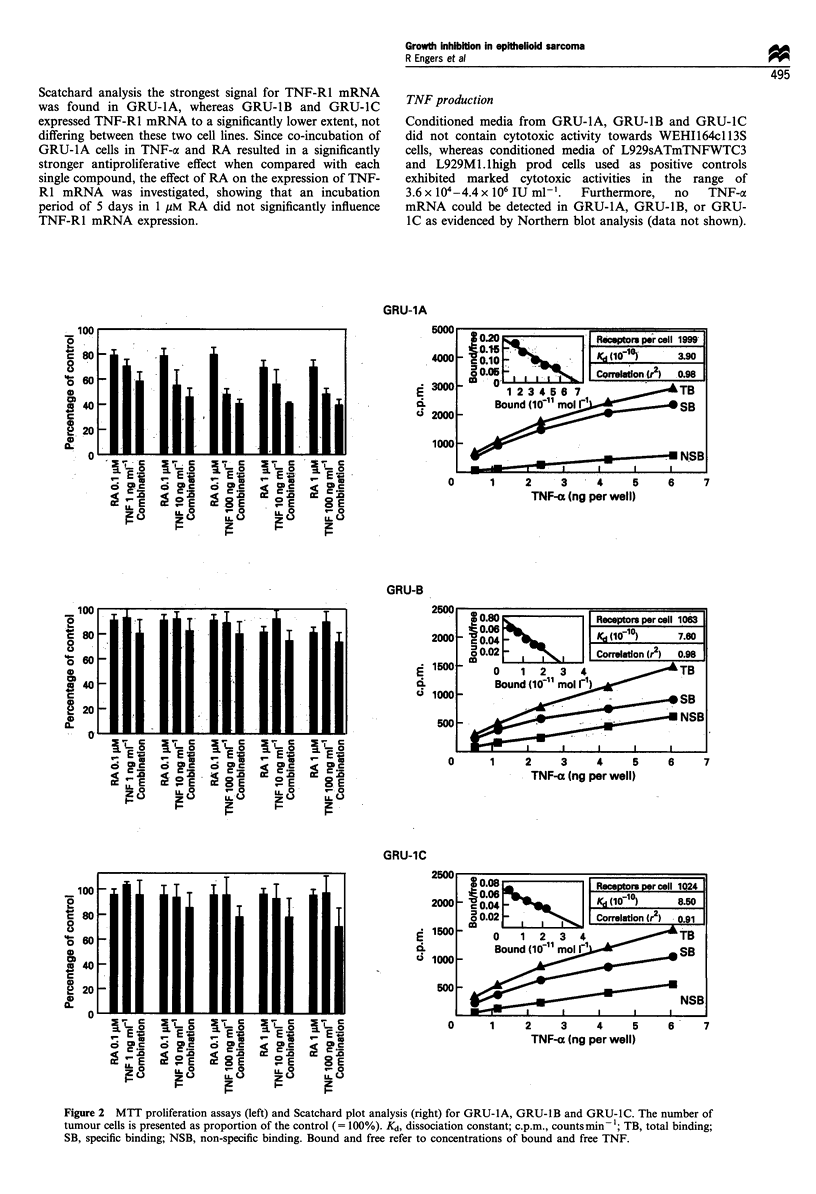
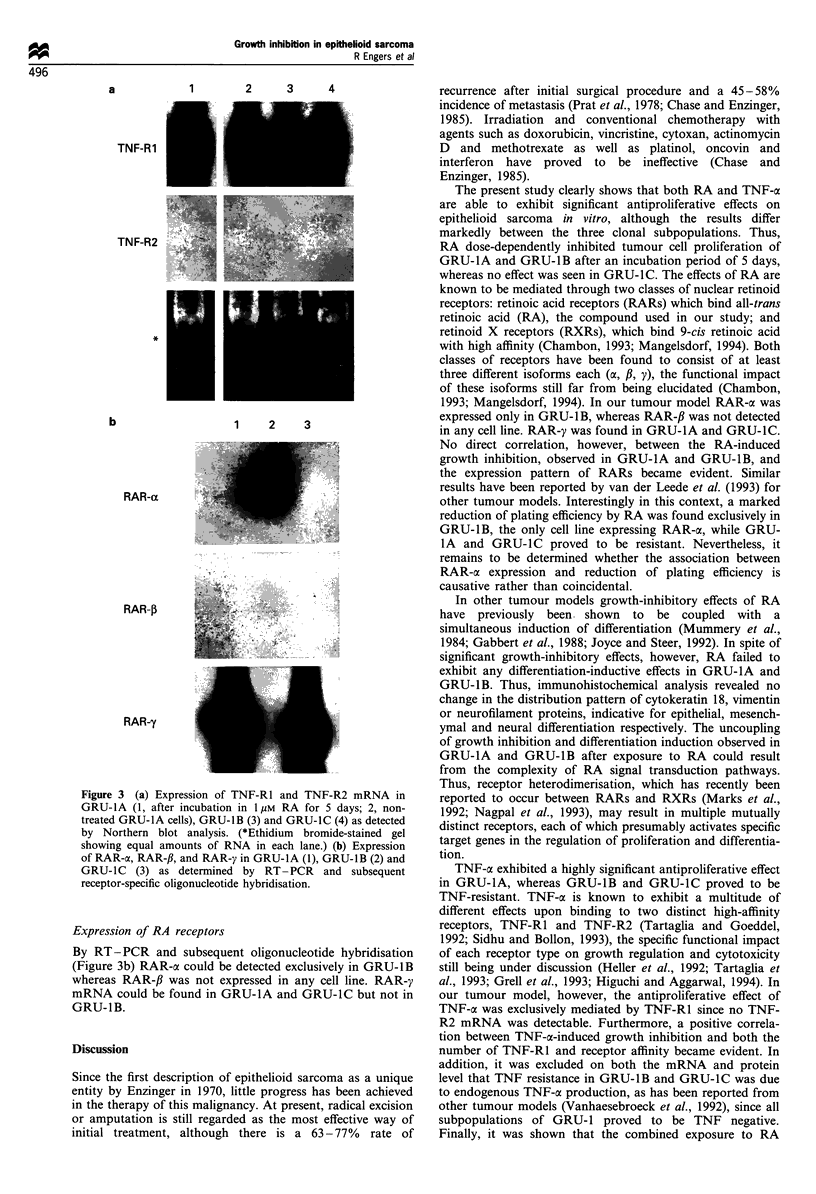
Epithelioid sarcoma is a highly malignant soft tissue tumour that is refractory to conventional chemotherapy and irradiation. Since permanent cell lines of this tumour are extremely rare, in vitro data on compounds with significant antiproliferative effects are still lacking. Therefore, we investigated the effects of retinoic acid (RA) and tumour necrosis factor alpha (TNF-alpha) on tumour cell proliferation of three different clonal subpopulations (GRU-1A, GRU-1B, GRU-1C) derived from the same human epithelioid sarcoma cell line, GRU-1. In GRU-1A both RA (P=0.01) and TNF-alpha (P=0.002) exhibited highly significant and dose-dependent growth inhibitory effects, which could further be increased by a combined application of both compounds (P<0.006). GRU-1B proved to be sensitive to RA (P=0.006), whereas no response to TNF-alpha was observed. GRU-1C was resistant to both RA and TNF-alpha. The antiproliferative effect of TNF-alpha was mediated by TNF receptor 1(TNF-R1) and correlated positively with both the number of TNF-R1 per cell and receptor affinity. No correlation was detected between RA-induced growth inhibition and the expression pattern of the RA receptors (RARs) RAR-alpha, RAR-beta, and RAR-gamma. Plating efficiency, however, could exclusively be reduced by RA in GRU-1B, the only cell line expressing RAR-alpha. Taken together, these data are the first showing significant antiproliferative effects in human epithelioid sarcoma by RA and TNF-alpha. Whereas the TNF-alpha response seems to depend on the expression of TNF-R1, no simple correlation could be found between RA sensitivity and the expression pattern of RARs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alley M. C., Scudiero D. A., Monks A., Hursey M. L., Czerwinski M. J., Fine D. L., Abbott B. J., Mayo J. G., Shoemaker R. H., Boyd M. R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988 Feb 1;48(3):589–601. [PubMed] [Google Scholar]
- Beyaert R., Fiers W. Molecular mechanisms of tumor necrosis factor-induced cytotoxicity. What we do understand and what we do not. FEBS Lett. 1994 Feb 28;340(1-2):9–16. doi: 10.1016/0014-5793(94)80163-0. [DOI] [PubMed] [Google Scholar]
- Chambon P. The molecular and genetic dissection of the retinoid signalling pathway. Gene. 1993 Dec 15;135(1-2):223–228. doi: 10.1016/0378-1119(93)90069-f. [DOI] [PubMed] [Google Scholar]
- Chase D. R., Enzinger F. M. Epithelioid sarcoma. Diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985 Apr;9(4):241–263. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25(2):193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Engers R., Gerharz C. D., Moll R., Pohl A., Sarbia M., Gabbert H. E. Interclonal heterogeneity in a human epithelioid-sarcoma cell line (GRU-1). Int J Cancer. 1994 Nov 15;59(4):548–553. doi: 10.1002/ijc.2910590419. [DOI] [PubMed] [Google Scholar]
- Enzinger F. M. Epitheloid sarcoma. A sarcoma simulating a granuloma or a carcinoma. Cancer. 1970 Nov;26(5):1029–1041. doi: 10.1002/1097-0142(197011)26:5<1029::aid-cncr2820260510>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ferrari N., Pfeffer U., Tosetti F., Brigati C., Vidali G. An improved RT-PCR protocol for the quantitation of human retinoic acid receptor RNA. Exp Cell Res. 1994 Mar;211(1):121–126. doi: 10.1006/excr.1994.1067. [DOI] [PubMed] [Google Scholar]
- Gabbert H. E., Gerharz C. D., Biesalski H. K., Engers R., Luley C. Terminal differentiation and growth inhibition of a rat rhabdomyosarcoma cell line (BA-HAN-1C) in vitro after exposure to retinoic acid. Cancer Res. 1988 Sep 15;48(18):5264–5269. [PubMed] [Google Scholar]
- Gerharz C. D., Bracke M. E., Mareel M. M., Gabbert H. E. Modulation of invasive potential in different clonal subpopulations of a rat rhabdomyosarcoma cell line (BA-HAN-1) by differentiation induction. Clin Exp Metastasis. 1993 Jan;11(1):55–67. doi: 10.1007/BF00880066. [DOI] [PubMed] [Google Scholar]
- Gerharz C. D., Moll R., Ramp U., Mellin W., Gabbert H. E. Multidirectional differentiation in a newly established human epithelioid sarcoma cell line (GRU-1) with co-expression of vimentin, cytokeratins and neurofilament proteins. Int J Cancer. 1990 Jan 15;45(1):143–152. doi: 10.1002/ijc.2910450126. [DOI] [PubMed] [Google Scholar]
- Grell M., Scheurich P., Meager A., Pfizenmaier K. TR60 and TR80 tumor necrosis factor (TNF)-receptors can independently mediate cytolysis. Lymphokine Cytokine Res. 1993 Jun;12(3):143–148. [PubMed] [Google Scholar]
- Halevy O., Lerman O. Retinoic acid induces adult muscle cell differentiation mediated by the retinoic acid receptor-alpha. J Cell Physiol. 1993 Mar;154(3):566–572. doi: 10.1002/jcp.1041540315. [DOI] [PubMed] [Google Scholar]
- Heller R. A., Song K., Fan N., Chang D. J. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell. 1992 Jul 10;70(1):47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Aggarwal B. B. Differential roles of two types of the TNF receptor in TNF-induced cytotoxicity, DNA fragmentation, and differentiation. J Immunol. 1994 Apr 15;152(8):4017–4025. [PubMed] [Google Scholar]
- Joyce D. A., Steer J. H. Differentiation of the U-937 promonocytic cell line induced by phorbol myristate acetate or retinoic acid: effect of aurothiomalate. Agents Actions. 1992 Nov;37(3-4):305–310. doi: 10.1007/BF02028124. [DOI] [PubMed] [Google Scholar]
- Lejeune F., Liénard D., Eggermont A., Schraffordt Koops H., Kroon B., Gérain J., Rosenkaimer F., Schmitz P. Clinical experience with high-dose tumor necrosis factor alpha in regional therapy of advanced melanoma. Circ Shock. 1994 Aug;43(4):191–197. [PubMed] [Google Scholar]
- Lejeune F., Liénard D., Eggermont A., Schraffordt Koops H., Rosenkaimer F., Gérain J., Klaase J., Kroon B., Vanderveken J., Schmitz P. Rationale for using TNF alpha and chemotherapy in regional therapy of melanoma. J Cell Biochem. 1994 Sep;56(1):52–61. doi: 10.1002/jcb.240560110. [DOI] [PubMed] [Google Scholar]
- Marks M. S., Hallenbeck P. L., Nagata T., Segars J. H., Appella E., Nikodem V. M., Ozato K. H-2RIIBP (RXR beta) heterodimerization provides a mechanism for combinatorial diversity in the regulation of retinoic acid and thyroid hormone responsive genes. EMBO J. 1992 Apr;11(4):1419–1435. doi: 10.1002/j.1460-2075.1992.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarvey T. W., Silberman S., Persky B. The effect of butyric acid and retinoic acid on invasion and experimental metastasis of murine melanoma cells. Clin Exp Metastasis. 1990 Sep-Oct;8(5):433–448. doi: 10.1007/BF00058154. [DOI] [PubMed] [Google Scholar]
- Moll R., Achtstätter T., Becht E., Balcarova-Ständer J., Ittensohn M., Franke W. W. Cytokeratins in normal and malignant transitional epithelium. Maintenance of expression of urothelial differentiation features in transitional cell carcinomas and bladder carcinoma cell culture lines. Am J Pathol. 1988 Jul;132(1):123–144. [PMC free article] [PubMed] [Google Scholar]
- Mummery C. L., van den Brink C. E., van der Saag P. T., de Laat S. W. The cell cycle, cell death, and cell morphology during retinoic acid-induced differentiation of embryonal carcinoma cells. Dev Biol. 1984 Aug;104(2):297–307. doi: 10.1016/0012-1606(84)90085-x. [DOI] [PubMed] [Google Scholar]
- Nagpal S., Friant S., Nakshatri H., Chambon P. RARs and RXRs: evidence for two autonomous transactivation functions (AF-1 and AF-2) and heterodimerization in vivo. EMBO J. 1993 Jun;12(6):2349–2360. doi: 10.1002/j.1460-2075.1993.tb05889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitz S., Moll R., Störkel S., Thoenes W. Expression of intermediate filament proteins in subtypes of renal cell carcinomas and in renal oncocytomas. Distinction of two classes of renal cell tumors. Lab Invest. 1987 Jun;56(6):642–653. [PubMed] [Google Scholar]
- Prat J., Woodruff J. M., Marcove R. C. Epithelioid sarcoma: an analysis of 22 cases indicating the prognostic significance of vascular invasion and regional lymph node metastasis. Cancer. 1978 Apr;41(4):1472–1487. doi: 10.1002/1097-0142(197804)41:4<1472::aid-cncr2820410436>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Pusztai L., Lewis C. E., McGee J. O. Growth arrest of the breast cancer cell line, T47D, by TNF alpha; cell cycle specificity and signal transduction. Br J Cancer. 1993 Feb;67(2):290–296. doi: 10.1038/bjc.1993.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves B. R., Fisher C., Smith S., Courtenay V. D., Robertson D. Ultrastructural, immunocytochemical, and cytogenetic characterization of a human epithelioid sarcoma cell line (RM-HS1). J Natl Cancer Inst. 1987 Jan;78(1):7–18. doi: 10.1093/jnci/78.1.7. [DOI] [PubMed] [Google Scholar]
- Rutka J. T., Giblin J. R., Berens M. E., Bar-Shiva E., Tokuda K., McCulloch J. R., Rosenblum M. L., Eessalu T. E., Aggarwal B. B., Bodell W. J. The effects of human recombinant tumor necrosis factor on glioma-derived cell lines: cellular proliferation, cytotoxicity, morphological and radioreceptor studies. Int J Cancer. 1988 Apr 15;41(4):573–582. doi: 10.1002/ijc.2910410417. [DOI] [PubMed] [Google Scholar]
- Scheurich P., Thoma B., Ucer U., Pfizenmaier K. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987 Mar 15;138(6):1786–1790. [PubMed] [Google Scholar]
- Scudiero D. A., Shoemaker R. H., Paull K. D., Monks A., Tierney S., Nofziger T. H., Currens M. J., Seniff D., Boyd M. R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988 Sep 1;48(17):4827–4833. [PubMed] [Google Scholar]
- Sidhu R. S., Bollon A. P. Tumor necrosis factor activities and cancer therapy--a perspective. Pharmacol Ther. 1993 Jan;57(1):79–128. doi: 10.1016/0163-7258(93)90037-e. [DOI] [PubMed] [Google Scholar]
- Sonobe H., Furihata M., Iwata J., Oka T., Ohtsuki Y., Hamasato S., Fujimoto S. Morphological characterization of a new human epithelioid sarcoma cell line, ES020488, in vitro and in vivo. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63(4):219–225. doi: 10.1007/BF02899265. [DOI] [PubMed] [Google Scholar]
- Stallcup M. R., Washington L. D. Region-specific initiation of mouse mammary tumor virus RNA synthesis by endogenous RNA polymerase II in preparations of cell nuclei. J Biol Chem. 1983 Mar 10;258(5):2802–2807. [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Goeddel D. V. Two TNF receptors. Immunol Today. 1992 May;13(5):151–153. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Rothe M., Hu Y. F., Goeddel D. V. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell. 1993 Apr 23;73(2):213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
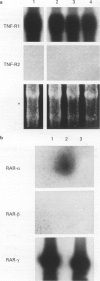
- Vanhaesebroeck B., Decoster E., Van Ostade X., Van Bladel S., Lenaerts A., Van Roy F., Fiers W. Expression of an exogenous tumor necrosis factor (TNF) gene in TNF-sensitive cell lines confers resistance to TNF-mediated cell lysis. J Immunol. 1992 May 1;148(9):2785–2794. [PubMed] [Google Scholar]
- Vanhaesebroeck B., Van Bladel S., Lenaerts A., Suffys P., Beyaert R., Lucas R., Van Roy F., Fiers W. Two discrete types of tumor necrosis factor-resistant cells derived from the same cell line. Cancer Res. 1991 May 1;51(9):2469–2477. [PubMed] [Google Scholar]
- van der Leede B. M., van den Brink C. E., van der Saag P. T. Retinoic acid receptor and retinoid X receptor expression in retinoic acid-resistant human tumor cell lines. Mol Carcinog. 1993;8(2):112–122. doi: 10.1002/mc.2940080208. [DOI] [PubMed] [Google Scholar]