Abstract
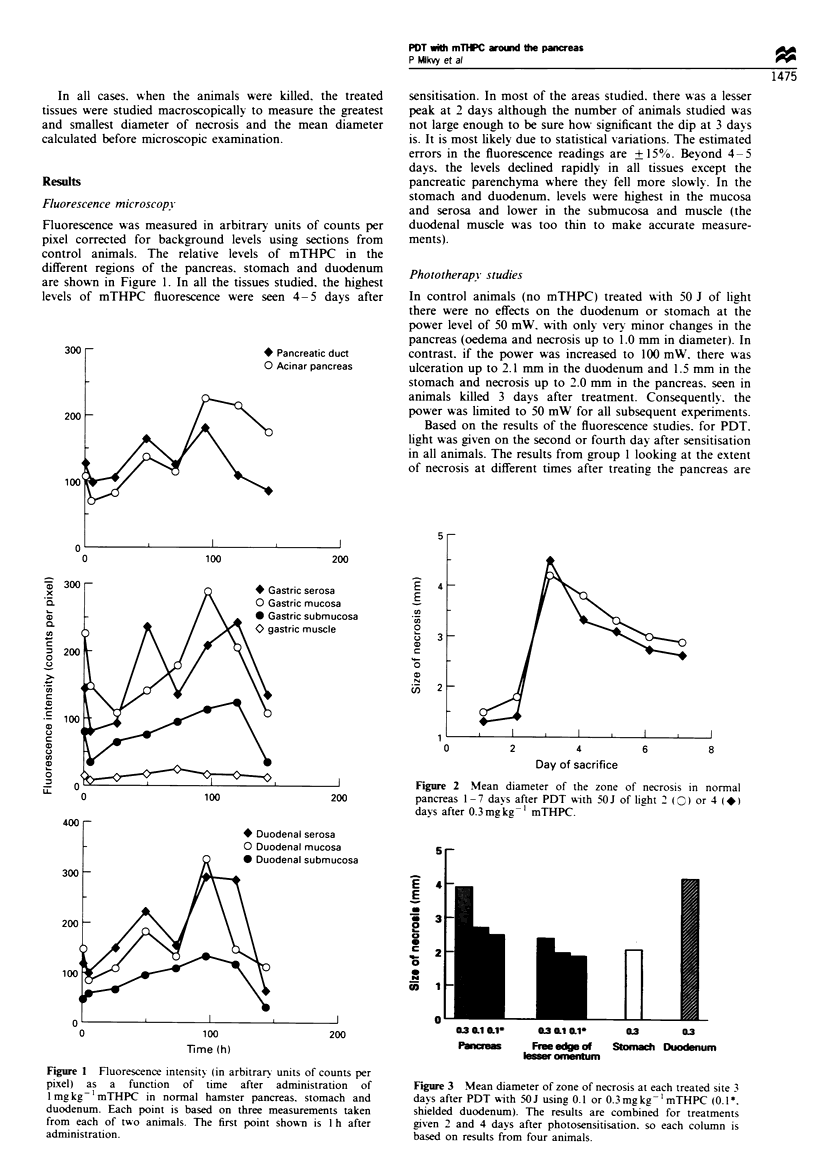
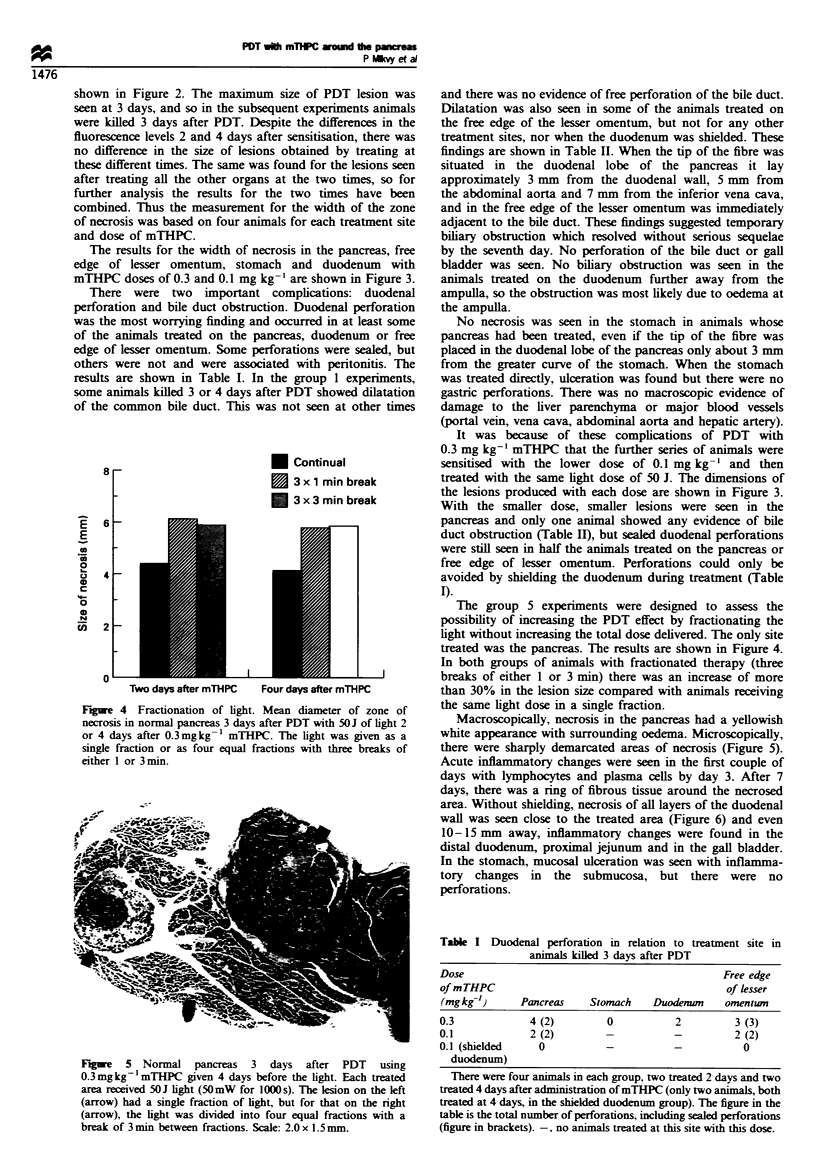
Photodynamic therapy (PDT) has the potential to destroy small tumours with safe healing of adjacent normal tissue. This study looks at the effects of PDT on the normal pancreas and adjacent tissues in hamsters using the photosensitiser meso-tetrahydroxyphenylchlorin (mTHPC). Pharmacokinetic studies used fluorescence microscopy on sections of pancreas, stomach and duodenum 1 h to 6 days after mTHPC. Highest levels of sensitiser were seen in the gastric and duodenal mucosa and in the acinar pancreas after 2-4 days. For PDT, light at 652 nm was delivered by placing a 0.2 mm diameter bare-ended fibre against the tissue. An energy of 50 J was used 2 or 4 days after 0.1 or 0.3 mg kg-1 mTHPC and animals killed 1 to 7 days later. Maximum necrosis was seen 3 days after PDT with lesions up to 4 mm in pancreas, 4.5 mm in duodenum and 2.5 mm in stomach. By fractionating the light dose, the lesion size could be increased by 30%. The main complication was free or sealed duodenal perforation (avoided by shielding the duodenum). Partial, reversible bile duct obstruction was seen occasionally. There was no macroscopic damage to the bile ducts or major blood vessels. Apart from the duodenum, all lesions healed safely. In this animal model, only the duodenum was at risk of serious, irreversible damage. Treatment is likely to be safer in the much thicker human duodenum. mTHPC is a powerful photosensitiser and suitable for further study for tumours in the region of the pancreas although care is required near the duodenum.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abulafi A. M., Allardice J. T., Williams N. S., van Someren N., Swain C. P., Ainley C. Photodynamic therapy for malignant tumours of the ampulla of Vater. Gut. 1995 Jun;36(6):853–856. doi: 10.1136/gut.36.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr H., Chatlani P., Tralau C. J., MacRobert A. J., Boulos P. B., Bown S. G. Local eradication of rat colon cancer with photodynamic therapy: correlation of distribution of photosensitiser with biological effects in normal and tumour tissue. Gut. 1991 May;32(5):517–523. doi: 10.1136/gut.32.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr H., Tralau C. J., Boulos P. B., MacRobert A. J., Krasner N., Phillips D., Bown S. G. Selective necrosis in dimethylhydrazine-induced rat colon tumors using phthalocyanine photodynamic therapy. Gastroenterology. 1990 Jun;98(6):1532–1537. doi: 10.1016/0016-5085(90)91086-l. [DOI] [PubMed] [Google Scholar]
- Barr H., Tralau C. J., Boulos P. B., MacRobert A. J., Tilly R., Bown S. G. The contrasting mechanisms of colonic collagen damage between photodynamic therapy and thermal injury. Photochem Photobiol. 1987 Nov;46(5):795–800. doi: 10.1111/j.1751-1097.1987.tb04850.x. [DOI] [PubMed] [Google Scholar]
- Berenbaum M. C., Akande S. L., Bonnett R., Kaur H., Ioannou S., White R. D., Winfield U. J. meso-Tetra(hydroxyphenyl)porphyrins, a new class of potent tumour photosensitisers with favourable selectivity. Br J Cancer. 1986 Nov;54(5):717–725. doi: 10.1038/bjc.1986.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnett R., Nizhnik A. N., White S. G., Berenbaum M. C. Porphyrin sensitizers in tumour phototherapy. Novel sensitizers of the chlorin and bacteriochlorin class with amphiphilic properties. J Photochem Photobiol B. 1990 Jun;6(1-2):29–37. doi: 10.1016/1011-1344(90)85071-4. [DOI] [PubMed] [Google Scholar]
- Bonnett R., White R. D., Winfield U. J., Berenbaum M. C. Hydroporphyrins of the meso-tetra(hydroxyphenyl)porphyrin series as tumour photosensitizers. Biochem J. 1989 Jul 1;261(1):277–280. doi: 10.1042/bj2610277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown S. G. Photodynamic therapy to scientists and clinicians--one world or two? J Photochem Photobiol B. 1990 Jun;6(1-2):1–12. doi: 10.1016/1011-1344(90)85069-9. [DOI] [PubMed] [Google Scholar]
- Bown S. G., Tralau C. J., Smith P. D., Akdemir D., Wieman T. J. Photodynamic therapy with porphyrin and phthalocyanine sensitisation: quantitative studies in normal rat liver. Br J Cancer. 1986 Jul;54(1):43–52. doi: 10.1038/bjc.1986.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. S., MacRobert A. J., Phillips D., Hart I. R. Use of charge coupled device camera for imaging of intracellular phthalocyanines. Photochem Photobiol. 1989 Nov;50(5):617–624. doi: 10.1111/j.1751-1097.1989.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Chan W. S., Marshall J. F., Svensen R., Bedwell J., Hart I. R. Effect of sulfonation on the cell and tissue distribution of the photosensitizer aluminum phthalocyanine. Cancer Res. 1990 Aug 1;50(15):4533–4538. [PubMed] [Google Scholar]
- Chatlani P. T., Bedwell J., MacRobert A. J., Barr H., Boulos P. B., Krasner N., Phillips D., Bown S. G. Comparison of distribution and photodynamic effects of di- and tetra-sulphonated aluminium phthalocyanines in normal rat colon. Photochem Photobiol. 1991 Jun;53(6):745–751. doi: 10.1111/j.1751-1097.1991.tb09887.x. [DOI] [PubMed] [Google Scholar]
- Chatlani P. T., Nuutinen P. J., Toda N., Barr H., MacRobert A. J., Bedwell J., Bown S. G. Selective necrosis in hamster pancreatic tumours using photodynamic therapy with phthalocyanine photosensitization. Br J Surg. 1992 Aug;79(8):786–790. doi: 10.1002/bjs.1800790826. [DOI] [PubMed] [Google Scholar]
- Li J. H., Guo Z. H., Jin M. L., Zhao F. Y., Cai W. M., Gao M. L., Shu M. Y., Zou J. Photodynamic therapy in the treatment of malignant tumours: an analysis of 540 cases. J Photochem Photobiol B. 1990 Jun;6(1-2):149–155. doi: 10.1016/1011-1344(90)85084-a. [DOI] [PubMed] [Google Scholar]
- Loh C. S., MacRobert A. J., Bedwell J., Regula J., Krasner N., Bown S. G. Oral versus intravenous administration of 5-aminolaevulinic acid for photodynamic therapy. Br J Cancer. 1993 Jul;68(1):41–51. doi: 10.1038/bjc.1993.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmann H., Mlkvy P., Buonaccorsi G., Davies C. L., MacRobert A. J., Bown S. G. Enhancement of photodynamic therapy with 5-aminolaevulinic acid-induced porphyrin photosensitisation in normal rat colon by threshold and light fractionation studies. Br J Cancer. 1995 Sep;72(3):589–594. doi: 10.1038/bjc.1995.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlkvy P., Messmann H., Debinski H., Regula J., Conio M., MacRobert A., Spigelman A., Phillips R., Bown S. G. Photodynamic therapy for polyps in familial adenomatous polyposis--a pilot study. Eur J Cancer. 1995 Jul-Aug;31A(7-8):1160–1165. doi: 10.1016/0959-8049(95)00276-o. [DOI] [PubMed] [Google Scholar]
- Nuutinen P. J., Chatlani P. T., Bedwell J., MacRobert A. J., Phillips D., Bown S. G. Distribution and photodynamic effect of disulphonated aluminium phthalocyanine in the pancreas and adjacent tissues in the Syrian golden hamster. Br J Cancer. 1991 Dec;64(6):1108–1115. doi: 10.1038/bjc.1991.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette B., Ali H., Langlois R., van Lier J. E. Biological activities of phthalocyanines--VIII. Cellular distribution in V-79 Chinese hamster cells and phototoxicity of selectively sulfonated aluminum phthalocyanines. Photochem Photobiol. 1988 Feb;47(2):215–220. doi: 10.1111/j.1751-1097.1988.tb02717.x. [DOI] [PubMed] [Google Scholar]
- Peng Q., Nesland J. M., Moan J., Evensen J. F., Kongshaug M., Rimington C. Localization of fluorescent Photofrin II and aluminum phthalocyanine tetrasulfonate in transplanted human malignant tumor LOX and normal tissues of nude mice using highly light-sensitive video intensification microscopy. Int J Cancer. 1990 May 15;45(5):972–979. doi: 10.1002/ijc.2910450533. [DOI] [PubMed] [Google Scholar]
- Regula J., Ravi B., Bedwell J., MacRobert A. J., Bown S. G. Photodynamic therapy using 5-aminolaevulinic acid for experimental pancreatic cancer--prolonged animal survival. Br J Cancer. 1994 Aug;70(2):248–254. doi: 10.1038/bjc.1994.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris H. B., Altermatt H. J., Inderbitzi R., Hess R., Nachbur B., Stewart J. C., Wang Q., Lim C. K., Bonnett R., Berenbaum M. C. Photodynamic therapy with chlorins for diffuse malignant mesothelioma: initial clinical results. Br J Cancer. 1991 Dec;64(6):1116–1120. doi: 10.1038/bjc.1991.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris H. B., Altermatt H. J., Nachbur B., Stewart J. C., Wang Q., Lim C. K., Bonnett R., Althaus U. Effect of drug-light interval on photodynamic therapy with meta-tetrahydroxyphenylchlorin in malignant mesothelioma. Int J Cancer. 1993 Jan 2;53(1):141–146. doi: 10.1002/ijc.2910530126. [DOI] [PubMed] [Google Scholar]
- Roberts W. G., Smith K. M., McCullough J. L., Berns M. W. Skin photosensitivity and photodestruction of several potential photodynamic sensitizers. Photochem Photobiol. 1989 Apr;49(4):431–438. doi: 10.1111/j.1751-1097.1989.tb09191.x. [DOI] [PubMed] [Google Scholar]
- Tralau C. J., Young A. R., Walker N. P., Vernon D. I., MacRobert A. J., Brown S. B., Bown S. G. Mouse skin photosensitivity with dihaematoporphyrin ether (DHE) and aluminium sulphonated phthalocyanine (AlSPc): a comparative study. Photochem Photobiol. 1989 Mar;49(3):305–312. doi: 10.1111/j.1751-1097.1989.tb04111.x. [DOI] [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- van der Veen N., van Leengoed H. L., Star W. M. In vivo fluorescence kinetics and photodynamic therapy using 5-aminolaevulinic acid-induced porphyrin: increased damage after multiple irradiations. Br J Cancer. 1994 Nov;70(5):867–872. doi: 10.1038/bjc.1994.412. [DOI] [PMC free article] [PubMed] [Google Scholar]




