Abstract
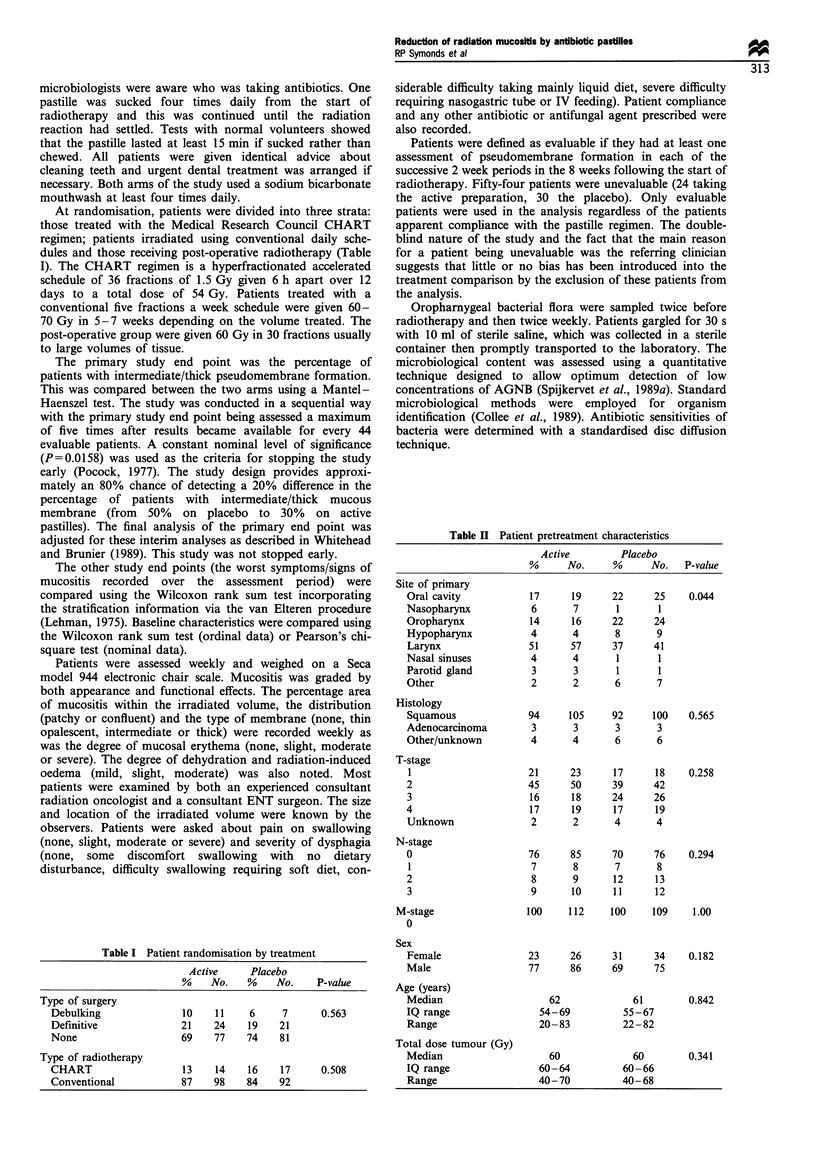
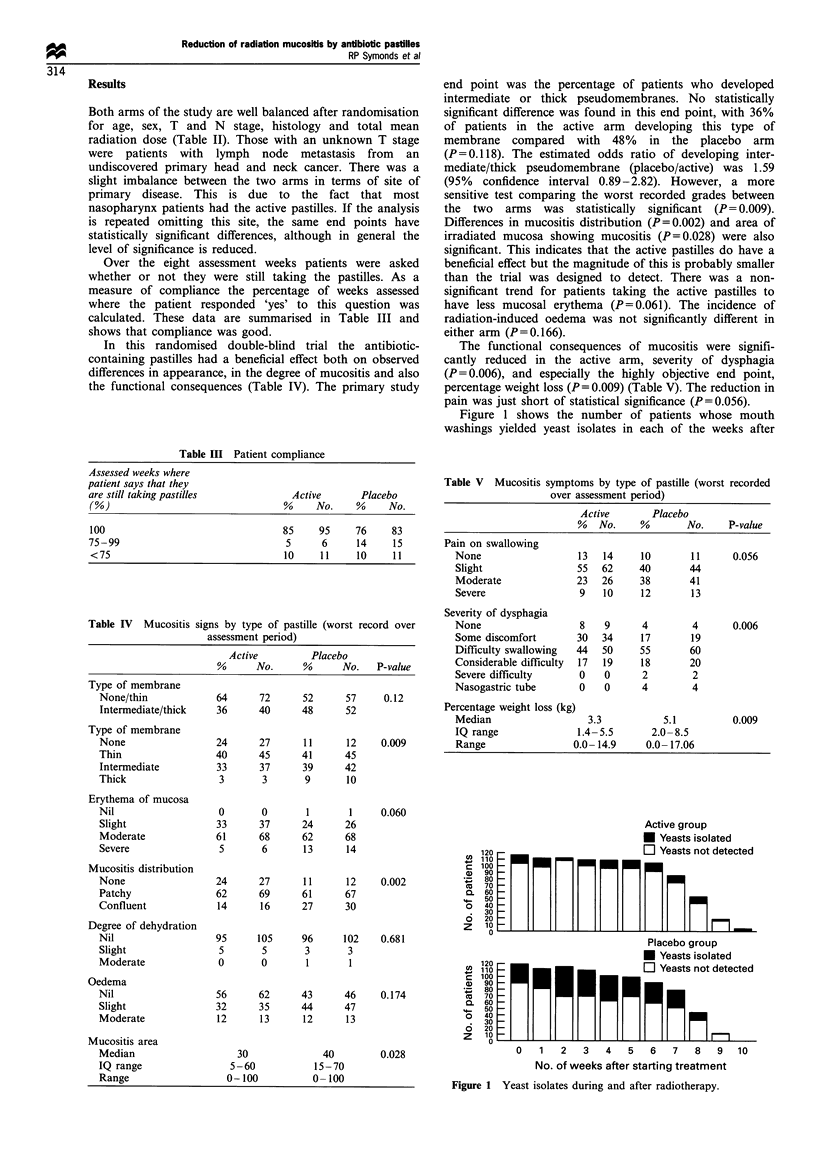
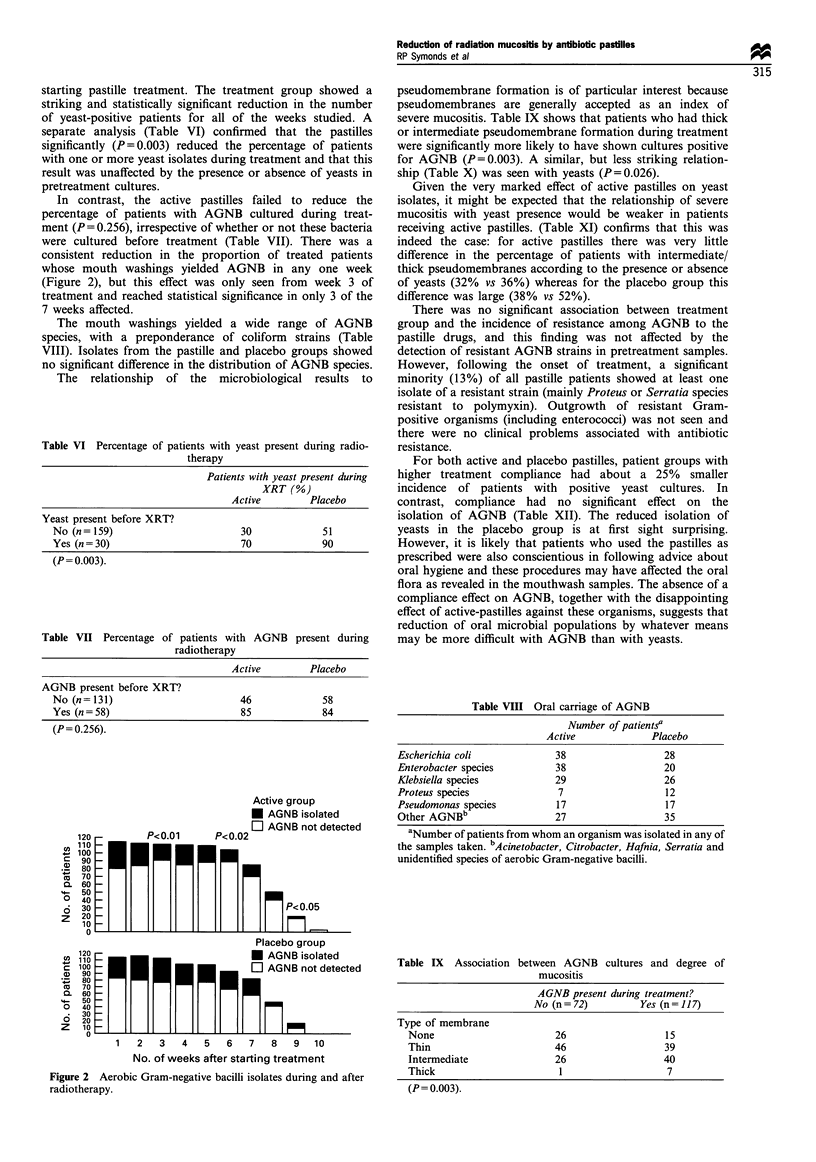
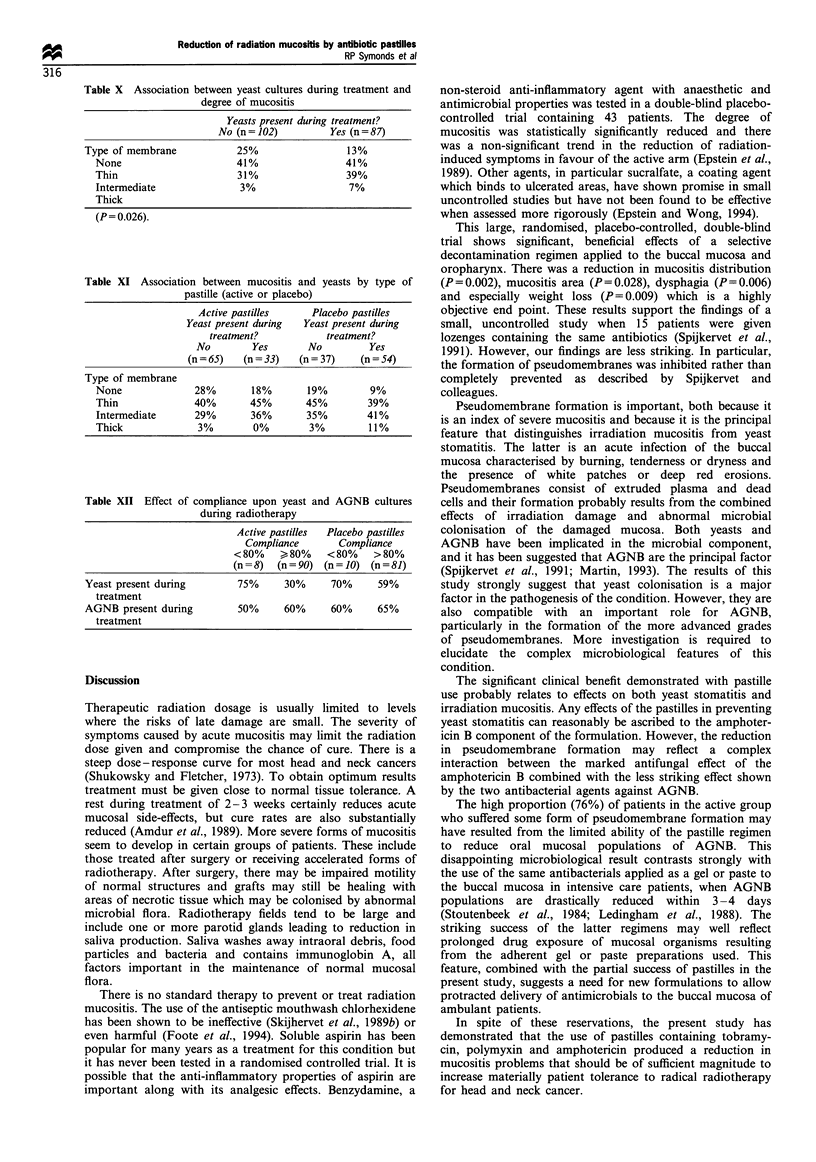
The aim of this study was to see if antibiotic pastilles could reduce radiation mucositis, pain, dysphagia and weight loss in patients undergoing radical radiotherapy for head and neck cancer. A total of 275 patients with T1-T4 tumours entered the study; 136 were allocated to suck four times daily a pastille containing amphotericin, polymyxin and tobramycin. The remaining 139 patients received an identical placebo. In all, 54 patients were unevaluable (24 active, 30 placebo). Bacteriological monitoring was carried out before and twice weekly during treatment. Both arms of the study were well balanced for T and N stage, age, sex and radiation dose (60 Gy). There was a slight imbalance in the site of disease which had no substantive effect on the results. The primary study end point was the percentage of patients who developed intermediate or thick pseudomembranes. No statistically significant difference was found in this end point, with 36% of patients in the active arm developing this type of membrane compared with 48% in the placebo arm (P = 0.118). The estimated odds ratio (placebo/active) of developing an intermediate or thick pseudomembrane was 1.59 (95% CI 0.89-2.82). However a more sensitive test comparing the worst recorded mucositis grade between the two arms was statistically significant (P = 0.009). This indicated that the active pastilles had a beneficial effect, but the magnitude was probably smaller than the trial was designed to detect. There was a reduction in mucositis distribution (P = 0.002), mucositis area (P = 0.028), dysphagia (P = 0.006) and weight loss (P = 0.009) in the active arm. There was a clear tendency for patients with positive cultures for aerobic Gram-negative bacteria (AGNB) (P = 0.003) and yeasts (P = 0.026) during treatment to have more severe mucositis. The active pastilles reduced the percentage of patients with yeast cultures (P = 0.003) but had less effect on AGNB. The benefit derived from the pastilles should materially increase patient tolerance to radical radiotherapy for head and neck cancer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amdur R. J., Parsons J. T., Mendenhall W. M., Million R. R., Cassisi N. J. Split-course versus continuous-course irradiation in the postoperative setting for squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1989 Aug;17(2):279–285. doi: 10.1016/0360-3016(89)90440-9. [DOI] [PubMed] [Google Scholar]
- Epstein J. B., Stevenson-Moore P., Jackson S., Mohamed J. H., Spinelli J. J. Prevention of oral mucositis in radiation therapy: a controlled study with benzydamine hydrochloride rinse. Int J Radiat Oncol Biol Phys. 1989 Jun;16(6):1571–1575. doi: 10.1016/0360-3016(89)90964-4. [DOI] [PubMed] [Google Scholar]
- Epstein J. B., Wong F. L. The efficacy of sucralfate suspension in the prevention of oral mucositis due to radiation therapy. Int J Radiat Oncol Biol Phys. 1994 Feb 1;28(3):693–698. doi: 10.1016/0360-3016(94)90195-3. [DOI] [PubMed] [Google Scholar]
- Foote R. L., Loprinzi C. L., Frank A. R., O'Fallon J. R., Gulavita S., Tewfik H. H., Ryan M. A., Earle J. M., Novotny P. Randomized trial of a chlorhexidine mouthwash for alleviation of radiation-induced mucositis. J Clin Oncol. 1994 Dec;12(12):2630–2633. doi: 10.1200/JCO.1994.12.12.2630. [DOI] [PubMed] [Google Scholar]
- Ledingham I. M., Alcock S. R., Eastaway A. T., McDonald J. C., McKay I. C., Ramsay G. Triple regimen of selective decontamination of the digestive tract, systemic cefotaxime, and microbiological surveillance for prevention of acquired infection in intensive care. Lancet. 1988 Apr 9;1(8589):785–790. doi: 10.1016/s0140-6736(88)91656-x. [DOI] [PubMed] [Google Scholar]
- Shukovsky L. J., Fletcher G. H. Time-dose and tumor volume relationships in the irradiation of squamous cell carcinoma of the tonsillar fossa. Radiology. 1973 Jun;107(3):621–626. doi: 10.1148/107.3.621. [DOI] [PubMed] [Google Scholar]
- Spijkervet F. K., van Saene H. K., Panders A. K., Vermey A., van Saene J. J., Mehta D. M., Fidler V. Effect of chlorhexidine rinsing on the oropharyngeal ecology in patients with head and neck cancer who have irradiation mucositis. Oral Surg Oral Med Oral Pathol. 1989 Feb;67(2):154–161. doi: 10.1016/0030-4220(89)90321-6. [DOI] [PubMed] [Google Scholar]
- Spijkervet F. K., van Saene H. K., van Saene J. J., Panders A. K., Vermey A., Mehta D. M. Mucositis prevention by selective elimination of oral flora in irradiated head and neck cancer patients. J Oral Pathol Med. 1990 Nov;19(10):486–489. doi: 10.1111/j.1600-0714.1990.tb00792.x. [DOI] [PubMed] [Google Scholar]
- Stoutenbeek C. P., van Saene H. K., Miranda D. R., Zandstra D. F. The effect of selective decontamination of the digestive tract on colonisation and infection rate in multiple trauma patients. Intensive Care Med. 1984;10(4):185–192. doi: 10.1007/BF00259435. [DOI] [PubMed] [Google Scholar]
- van Saene H. K., Martin M. V. Do microorganisms play a role in irradiation mucositis? Eur J Clin Microbiol Infect Dis. 1990 Dec;9(12):861–863. doi: 10.1007/BF01967499. [DOI] [PubMed] [Google Scholar]


