Abstract
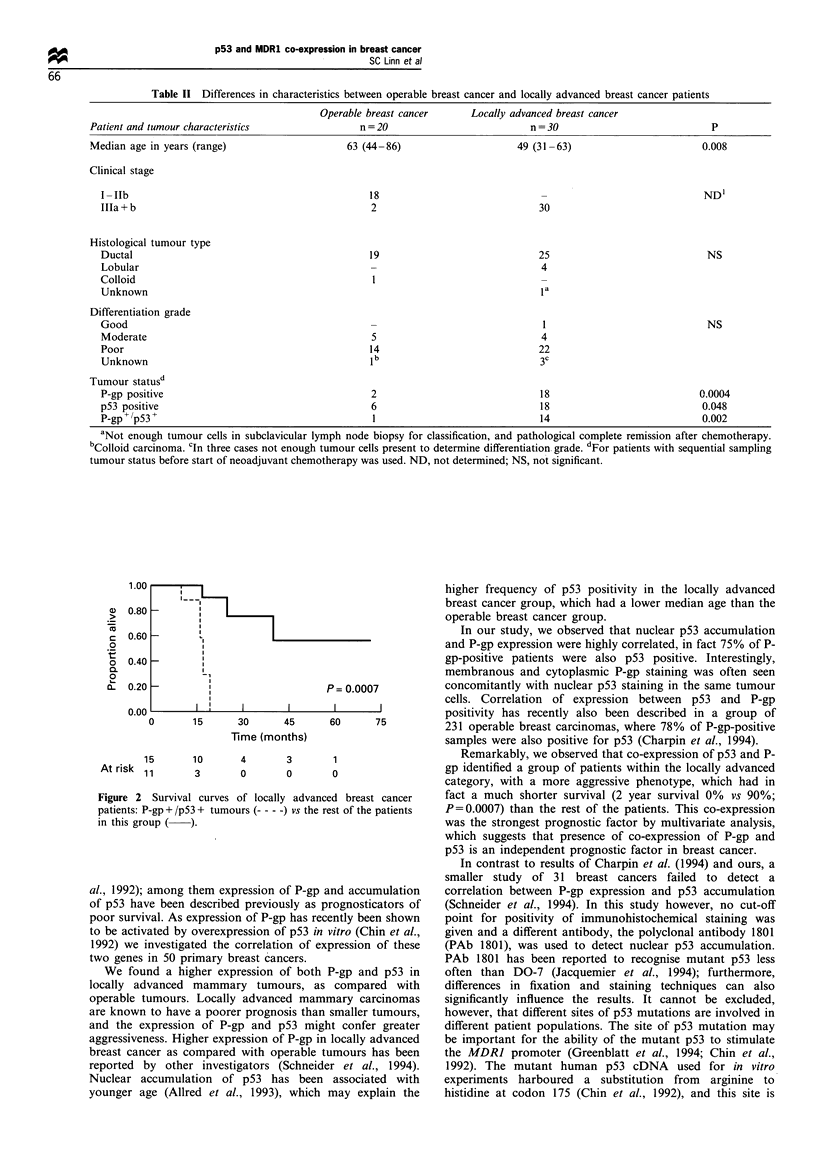
Expression of both P-glycoprotein (P-gp) and mutant p53 have recently been reported to be associated with poor prognosis of breast cancer. The expression of P-gp is associated in vitro and in vivo with cross-resistance to several anti-cancer drugs. p53 plays a regulatory role in apoptosis, and mutant p53 has been suggested to be involved in drug resistance. Interestingly, in vitro experiments have shown that mutant p53 can activate the promoter of the MDR1 gene, which encodes P-gp. We investigated whether p53 and P-gp are simultaneously expressed in primary breast cancer cells and analysed the impact of the co-expression on patients prognosis. Immunohistochemistry was used to investigate P-gp expression (JSB-1, C219) and nuclear p53 accumulation (DO-7) in 20 operable chemotherapy untreated and 30 locally advanced breast cancers undergoing neoadjuvant chemotherapy with doxorubicin and cyclophosphamide. Double immunostaining showed that P-gp expression and nuclear p53 accumulation often occur concomitantly in the same tumour cells. A correlation between p53 and P-gp expression was found in all 50 breast cancers (P = 0.003; Fisher's exact test). P-gp expression, nuclear p53 accumulation, and co-expression of p53 and P-gp were more frequently observed in locally advanced breast cancers than in operable breast cancers (P = 0.0004, P = 0.048; P = 0.002 respectively. Fisher's exact test). Co-expression of p53 and P-gp was the strongest prognostic factor for shorter survival by multivariate analysis (P = 0.004) in the group of locally advanced breast cancers (univariate analysis: P = 0.0007). Only 3 out of 13 samples sequentially taken before and after chemotherapy displayed a change in P-gp or p53 staining. In conclusion, nuclear p53 accumulation is often associated with P-gp expression in primary breast cancer, and simultaneous expression of p53 and P-gp is associated with shorter survival in locally advanced breast cancer patients. Co-expression of P-gp and mutant p53 belong to a series of molecular events resulting in a more aggressive phenotype, drug resistance and poor prognosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. C., Clark G. M., Elledge R., Fuqua S. A., Brown R. W., Chamness G. C., Osborne C. K., McGuire W. L. Association of p53 protein expression with tumor cell proliferation rate and clinical outcome in node-negative breast cancer. J Natl Cancer Inst. 1993 Feb 3;85(3):200–206. doi: 10.1093/jnci/85.3.200. [DOI] [PubMed] [Google Scholar]
- Chan H. S., Haddad G., Thorner P. S., DeBoer G., Lin Y. P., Ondrusek N., Yeger H., Ling V. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N Engl J Med. 1991 Dec 5;325(23):1608–1614. doi: 10.1056/NEJM199112053252304. [DOI] [PubMed] [Google Scholar]
- Charpin C., Vielh P., Duffaud F., Devictor B., Andrac L., Lavaut M. N., Allasia C., Horschowski N., Piana L. Quantitative immunocytochemical assays of P-glycoprotein in breast carcinomas: correlation to messenger RNA expression and to immunohistochemical prognostic indicators. J Natl Cancer Inst. 1994 Oct 19;86(20):1539–1545. doi: 10.1093/jnci/86.20.1539. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Ueda K., Pastan I., Gottesman M. M. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992 Jan 24;255(5043):459–462. doi: 10.1126/science.1346476. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Grimm E. A., Mukhopadhyay T., Zhang W. W., Owen-Schaub L. B., Roth J. A. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Res. 1994 May 1;54(9):2287–2291. [PubMed] [Google Scholar]
- Greenblatt M. S., Bennett W. P., Hollstein M., Harris C. C. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994 Sep 15;54(18):4855–4878. [PubMed] [Google Scholar]
- Harris J. R., Lippman M. E., Veronesi U., Willett W. Breast cancer (2). N Engl J Med. 1992 Aug 6;327(6):390–398. doi: 10.1056/NEJM199208063270606. [DOI] [PubMed] [Google Scholar]
- Hoekman K., Wagstaff J., van Groeningen C. J., Vermorken J. B., Boven E., Pinedo H. M. Effects of recombinant human granulocyte-macrophage colony-stimulating factor on myelosuppression induced by multiple cycles of high-dose chemotherapy in patients with advanced breast cancer. J Natl Cancer Inst. 1991 Nov 6;83(21):1546–1553. doi: 10.1093/jnci/83.21.1546. [DOI] [PubMed] [Google Scholar]
- Jacquemier J., Molès J. P., Penault-Llorca F., Adélaide J., Torrente M., Viens P., Birnbaum D., Theillet C. p53 immunohistochemical analysis in breast cancer with four monoclonal antibodies: comparison of staining and PCR-SSCP results. Br J Cancer. 1994 May;69(5):846–852. doi: 10.1038/bjc.1994.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith W. N., Stallard S., Brown R. Expression of mdr1 and gst-pi in human breast tumours: comparison to in vitro chemosensitivity. Br J Cancer. 1990 May;61(5):712–716. doi: 10.1038/bjc.1990.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. W., Bodis S., McClatchey A., Remington L., Ruley H. E., Fisher D. E., Housman D. E., Jacks T. p53 status and the efficacy of cancer therapy in vivo. Science. 1994 Nov 4;266(5186):807–810. doi: 10.1126/science.7973635. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Mullink H., Henzen-Logmans S. C., Alons-van Kordelaar J. J., Tadema T. M., Meijer C. J. Simultaneous immunoenzyme staining of vimentin and cytokeratins with monoclonal antibodies as an aid in the differential diagnosis of malignant mesothelioma from pulmonary adenocarcinoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(1):55–65. doi: 10.1007/BF02889950. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Grogan T. M., Miller T., Scheper R., Dalton W. S. Prediction of doxorubicin resistance in vitro in myeloma, lymphoma, and breast cancer by P-glycoprotein staining. J Natl Cancer Inst. 1989 May 3;81(9):696–701. doi: 10.1093/jnci/81.9.696. [DOI] [PubMed] [Google Scholar]
- Sanfilippo O., Ronchi E., De Marco C., Di Fronzo G., Silvestrini R. Expression of P-glycoprotein in breast cancer tissue and in vitro resistance to doxorubicin and vincristine. Eur J Cancer. 1991;27(2):155–158. doi: 10.1016/0277-5379(91)90476-t. [DOI] [PubMed] [Google Scholar]
- Scheper R. J., Bulte J. W., Brakkee J. G., Quak J. J., van der Schoot E., Balm A. J., Meijer C. J., Broxterman H. J., Kuiper C. M., Lankelma J. Monoclonal antibody JSB-1 detects a highly conserved epitope on the P-glycoprotein associated with multi-drug-resistance. Int J Cancer. 1988 Sep 15;42(3):389–394. doi: 10.1002/ijc.2910420314. [DOI] [PubMed] [Google Scholar]
- Schneider J., Bak M., Efferth T., Kaufmann M., Mattern J., Volm M. P-glycoprotein expression in treated and untreated human breast cancer. Br J Cancer. 1989 Dec;60(6):815–818. doi: 10.1038/bjc.1989.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Rubio M. P., Barbazán M. J., Rodriguez-Escudero F. J., Seizinger B. R., Castresana J. S. P-glycoprotein, HER-2/neu, and mutant p53 expression in human gynecologic tumors. J Natl Cancer Inst. 1994 Jun 1;86(11):850–855. doi: 10.1093/jnci/86.11.850. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Thor A. D., Moore DH I. I., Edgerton S. M., Kawasaki E. S., Reihsaus E., Lynch H. T., Marcus J. N., Schwartz L., Chen L. C., Mayall B. H. Accumulation of p53 tumor suppressor gene protein: an independent marker of prognosis in breast cancers. J Natl Cancer Inst. 1992 Jun 3;84(11):845–855. doi: 10.1093/jnci/84.11.845. [DOI] [PubMed] [Google Scholar]
- Verrelle P., Meissonnier F., Fonck Y., Feillel V., Dionet C., Kwiatkowski F., Plagne R., Chassagne J. Clinical relevance of immunohistochemical detection of multidrug resistance P-glycoprotein in breast carcinoma. J Natl Cancer Inst. 1991 Jan 16;83(2):111–116. doi: 10.1093/jnci/83.2.111. [DOI] [PubMed] [Google Scholar]
- Wishart G. C., Plumb J. A., Going J. J., McNicol A. M., McArdle C. S., Tsuruo T., Kaye S. B. P-glycoprotein expression in primary breast cancer detected by immunocytochemistry with two monoclonal antibodies. Br J Cancer. 1990 Nov;62(5):758–761. doi: 10.1038/bjc.1990.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kalken C. K., Pinedo H. M., Giaccone G. Multidrug resistance from the clinical point of view. Eur J Cancer. 1991;27(11):1481–1486. doi: 10.1016/0277-5379(91)90036-d. [DOI] [PubMed] [Google Scholar]
- van der Valk P., van Kalken C. K., Ketelaars H., Broxterman H. J., Scheffer G., Kuiper C. M., Tsuruo T., Lankelma J., Meijer C. J., Pinedo H. M. Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein molecule. Ann Oncol. 1990;1(1):56–64. [PubMed] [Google Scholar]



