Abstract
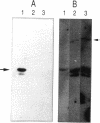
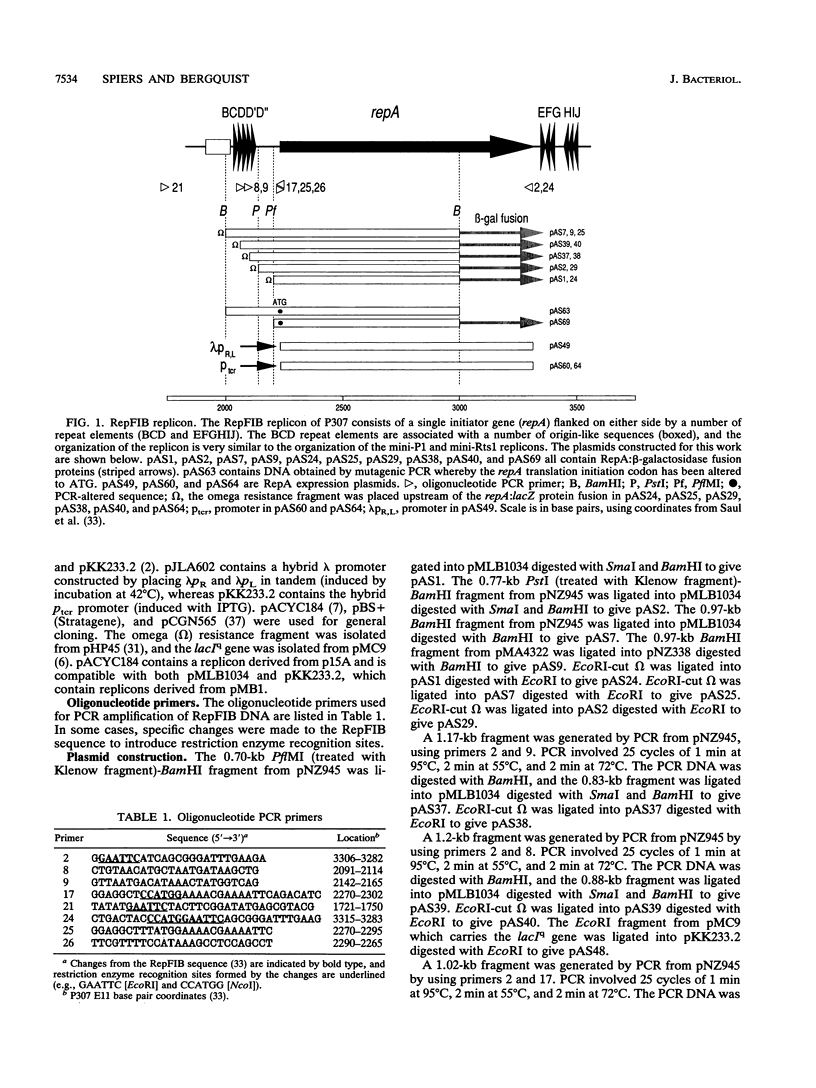
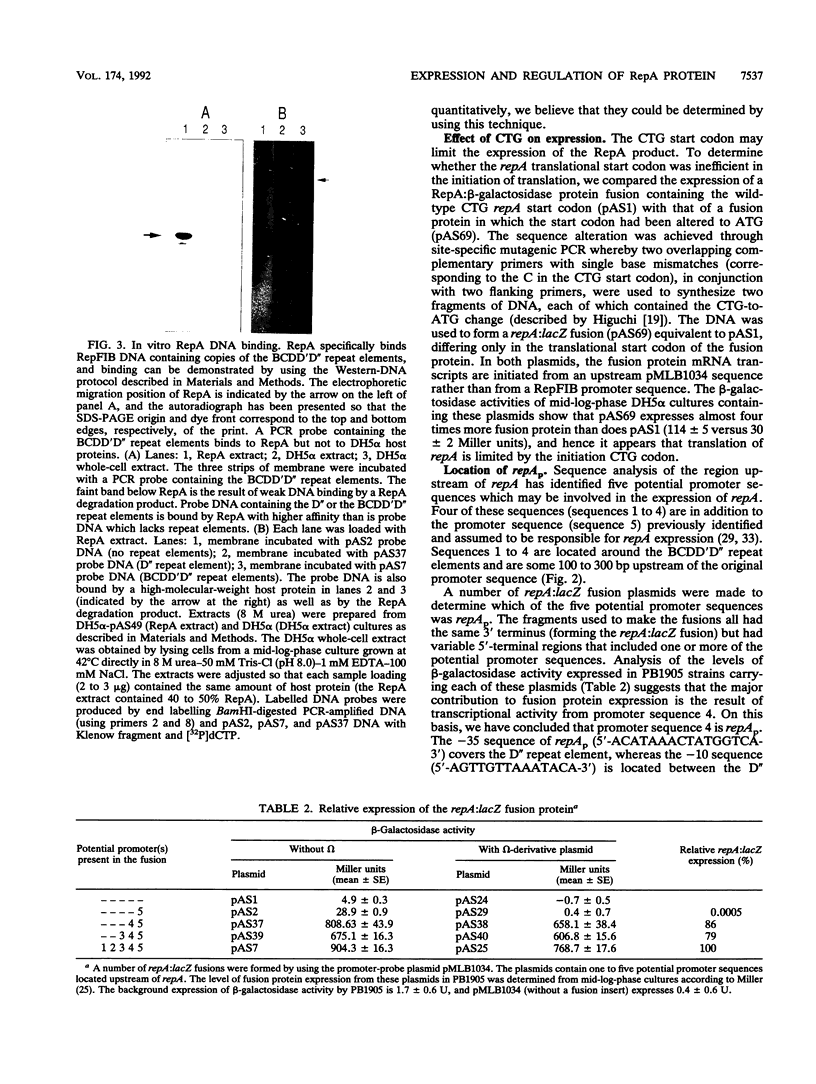
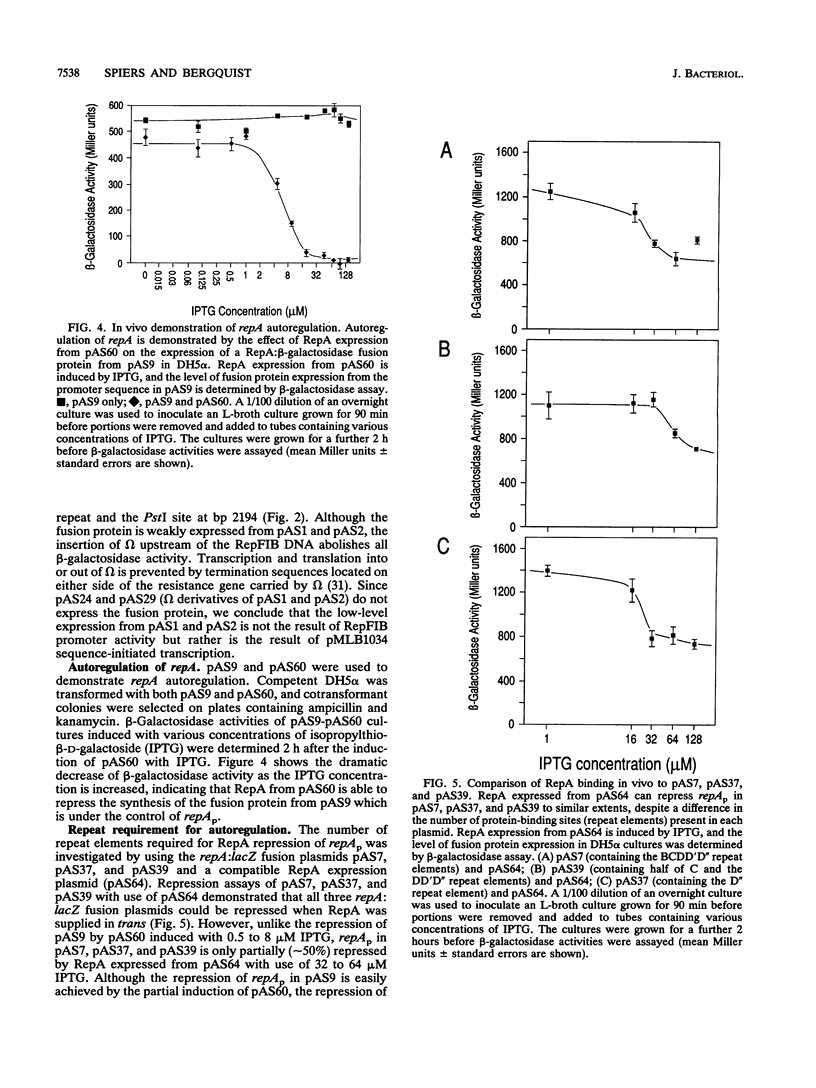
The control of RepFIB replication appears to rely on the interaction between an initiator protein (RepA) and two sets of DNA repeat elements located on either side of the repA gene. Limited N-terminal sequence information obtained from a RepA:beta-galactosidase fusion protein indicates that although the first residue of RepA is methionine, the initiation of translation of RepA occurs from a CTG codon rather than from the predicted GTG codon located further downstream. Overexpressed RepA in trans is capable of repressing a repA:lacZ fusion plasmid in which the expression of the fusion protein is under the control of the repA promoter. The repA promoter has been located functionally by testing a series of repA:lacZ fusion plasmids. Both in vivo genetic tests and in vitro DNA-binding studies indicate that repA autoregulation can be achieved by RepA binding to one or more repeat elements which overlap the repA promoter sequence.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L., Snyder K. M., Chattoraj D. K. P1 plasmid replication: replicon structure. J Mol Biol. 1984 Mar 5;173(3):307–324. doi: 10.1016/0022-2836(84)90123-2. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Bergquist P. L., Lane H. E., Malcolm L., Downard R. A. Molecular homology and incompatibility in the IncFI plasmid Group. J Gen Microbiol. 1982 Feb;128(2):223–238. doi: 10.1099/00221287-128-2-223. [DOI] [PubMed] [Google Scholar]
- Bergquist P. L., Saadi S., Maas W. K. Distribution of basic replicons having homology with RepFIA, RepFIB, and RepFIC among IncF group plasmids. Plasmid. 1986 Jan;15(1):19–34. doi: 10.1016/0147-619x(86)90011-9. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., McEachern M., Greener A., Mukhopadhyay P., Uhlenhopp E., Durland R., Helinski D. Role of the pi initiation protein and direct nucleotide sequence repeats in the regulation of plasmid R6K replication. Basic Life Sci. 1985;30:125–140. doi: 10.1007/978-1-4613-2447-8_13. [DOI] [PubMed] [Google Scholar]
- Froehlich B. J., Scott J. R. A single amino acid difference between Rep proteins of P1 and P7 affects plasmid copy number. Plasmid. 1988 Mar;19(2):121–133. doi: 10.1016/0147-619x(88)90051-0. [DOI] [PubMed] [Google Scholar]
- Gammie A. E., Crosa J. H. Co-operative autoregulation of a replication protein gene. Mol Microbiol. 1991 Dec;5(12):3015–3023. doi: 10.1111/j.1365-2958.1991.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Gardner R., McAnulty J., Feher E., Lane D. Location of rep and inc sequences in the F secondary replicon. Plasmid. 1985 Mar;13(2):145–148. doi: 10.1016/0147-619x(85)90067-8. [DOI] [PubMed] [Google Scholar]
- Gold L. Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- Hartz D., Binkley J., Hollingsworth T., Gold L. Domains of initiator tRNA and initiation codon crucial for initiator tRNA selection by Escherichia coli IF3. Genes Dev. 1990 Oct;4(10):1790–1800. doi: 10.1101/gad.4.10.1790. [DOI] [PubMed] [Google Scholar]
- Hartz D., McPheeters D. S., Gold L. Selection of the initiator tRNA by Escherichia coli initiation factors. Genes Dev. 1989 Dec;3(12A):1899–1912. doi: 10.1101/gad.3.12a.1899. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlt M., Schwarz H. P., Neumann R., Eggers H. J. Detection of DNA-protein binding in western blots by phosphorus-labeled and biotinylated DNA probes. Biotechniques. 1988 Apr;6(4):324–331. [PubMed] [Google Scholar]
- Kamio Y., Itoh Y., Terawaki Y. Purification of Rts1 RepA protein and binding of the protein to mini-Rts1 DNA. J Bacteriol. 1988 Sep;170(9):4411–4414. doi: 10.1128/jb.170.9.4411-4414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Terawaki Y. Nucleotide sequence of an incompatibility region of mini-Rts1 that contains five direct repeats. J Bacteriol. 1983 Sep;155(3):1185–1191. doi: 10.1128/jb.155.3.1185-1191.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D., Gardner R. C. Second EcoRI fragment of F capable of self-replication. J Bacteriol. 1979 Jul;139(1):141–151. doi: 10.1128/jb.139.1.141-151.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelong J. C., Prevost G., Lee K., Crepin M. South western blot mapping: a procedure for simultaneous characterization of DNA binding proteins and their specific genomic DNA target sites. Anal Biochem. 1989 Jun;179(2):299–303. doi: 10.1016/0003-2697(89)90132-2. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Limited N-terminal sequence analysis. Methods Enzymol. 1990;182:602–613. doi: 10.1016/0076-6879(90)82047-6. [DOI] [PubMed] [Google Scholar]
- Nordström K. Control of plasmid replication: theoretical considerations and practical solutions. Basic Life Sci. 1985;30:189–214. doi: 10.1007/978-1-4613-2447-8_17. [DOI] [PubMed] [Google Scholar]
- Nozue H., Tsuchiya K., Kamio Y. Nucleotide sequence and copy control function of the extension of the incI region (incI-b) of Rts 1. Plasmid. 1988 Jan;19(1):46–56. doi: 10.1016/0147-619x(88)90062-5. [DOI] [PubMed] [Google Scholar]
- Perez-Casal J. F., Crosa J. H. Novel incompatibility and partition loci for the REPI replication region of plasmid ColV-K30. J Bacteriol. 1987 Nov;169(11):5078–5086. doi: 10.1128/jb.169.11.5078-5086.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Casal J. F., Gammie A. E., Crosa J. H. Nucleotide sequence analysis and expression of the minimum REPI replication region and incompatibility determinants of pColV-K30. J Bacteriol. 1989 Apr;171(4):2195–2201. doi: 10.1128/jb.171.4.2195-2201.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Romero A., García P. Initiation of translation at AUC, AUA and AUU codons in Escherichia coli. FEMS Microbiol Lett. 1991 Dec 1;68(3):325–330. doi: 10.1016/0378-1097(91)90377-m. [DOI] [PubMed] [Google Scholar]
- Saul D., Spiers A. J., McAnulty J., Gibbs M. G., Bergquist P. L., Hill D. F. Nucleotide sequence and replication characteristics of RepFIB, a basic replicon of IncF plasmids. J Bacteriol. 1989 May;171(5):2697–2707. doi: 10.1128/jb.171.5.2697-2707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauder B., Blöcker H., Frank R., McCarthy J. E. Inducible expression vectors incorporating the Escherichia coli atpE translational initiation region. Gene. 1987;52(2-3):279–283. doi: 10.1016/0378-1119(87)90054-0. [DOI] [PubMed] [Google Scholar]
- Shinedling S., Gayle M., Pribnow D., Gold L. Mutations affecting translation of the bacteriophage T4 rIIB gene cloned in Escherichia coli. Mol Gen Genet. 1987 May;207(2-3):224–232. doi: 10.1007/BF00331582. [DOI] [PubMed] [Google Scholar]
- Shultz J., Silhavy T. J., Berman M. L., Fiil N., Emr S. D. A previously unidentified gene in the spc operon of Escherichia coli K12 specifies a component of the protein export machinery. Cell. 1982 Nov;31(1):227–235. doi: 10.1016/0092-8674(82)90422-6. [DOI] [PubMed] [Google Scholar]
- Stalker D. M., McBride K. E., Malyj L. D. Herbicide resistance in transgenic plants expressing a bacterial detoxification gene. Science. 1988 Oct 21;242(4877):419–423. doi: 10.1126/science.242.4877.419. [DOI] [PubMed] [Google Scholar]
- Tabuchi A. Nucleotide sequence of the replication region of plasmid R401 and its incompatibility function. Microbiol Immunol. 1985;29(5):383–393. doi: 10.1111/j.1348-0421.1985.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W., Gualerzi C. Effect of Escherichia coli initiation factors on the kinetics of N-Acphe-tRNAPhe binding to 30S ribosomal subunits. A fluorescence stopped-flow study. Biochemistry. 1983 Feb 1;22(3):690–694. doi: 10.1021/bi00272a025. [DOI] [PubMed] [Google Scholar]