Abstract
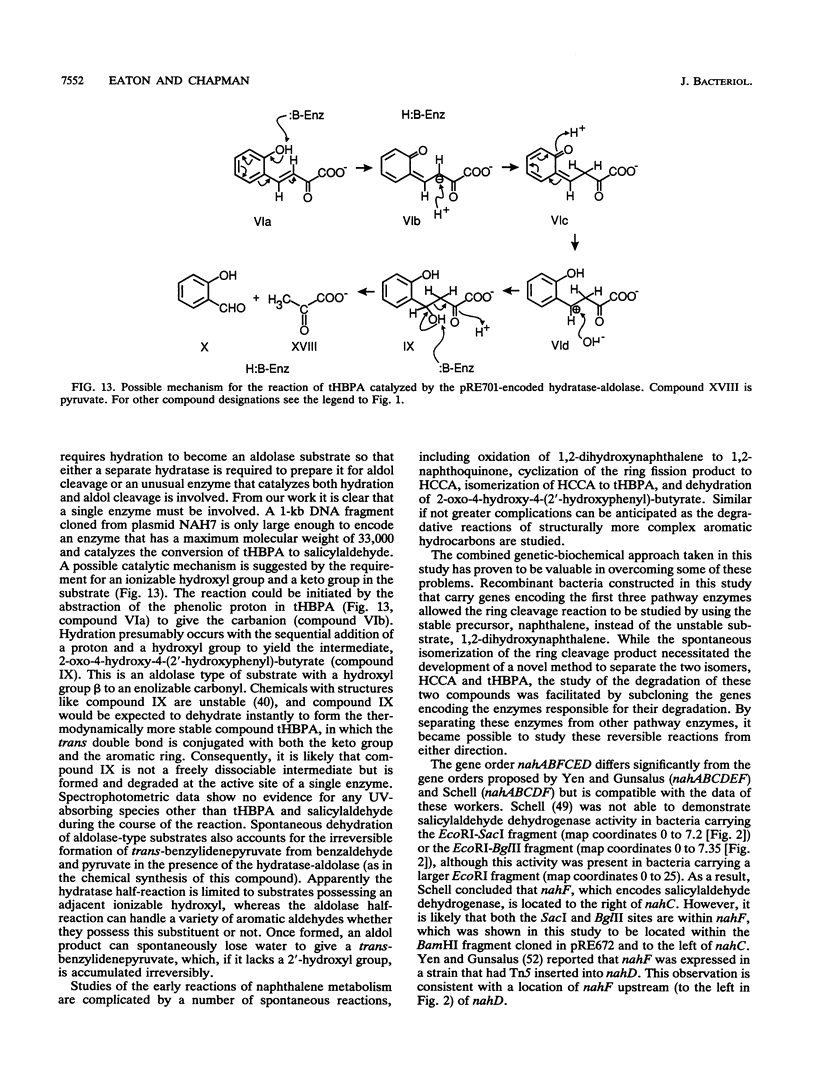
The reactions involved in the bacterial metabolism of naphthalene to salicylate have been reinvestigated by using recombinant bacteria carrying genes cloned from plasmid NAH7. When intact cells of Pseudomonas aeruginosa PAO1 carrying DNA fragments encoding the first three enzymes of the pathway were incubated with naphthalene, they formed products of the dioxygenase-catalyzed ring cleavage of 1,2-dihydroxynaphthalene. These products were separated by chromatography on Sephadex G-25 and were identified by 1H and 13C nuclear magnetic resonance spectroscopy and gas chromatography-mass spectrometry as 2-hydroxychromene-2-carboxylate (HCCA) and trans-o-hydroxybenzylidenepyruvate (tHBPA). HCCA was detected as the first reaction product in these incubation mixtures by its characteristic UV spectrum, which slowly changed to a spectrum indicative of an equilibrium mixture of HCCA and tHBPA. Isomerization of either purified product occurred slowly and spontaneously to give an equilibrium mixture of essentially the same composition. tHBPA is also formed from HCCA by the action of an isomerase enzyme encoded by plasmid NAH7. The gene encoding this enzyme, nahD, was cloned on a 1.95-kb KpnI-BglII fragment. Extracts of Escherichia coli JM109 carrying this fragment catalyzed the rapid equilibration of HCCA and tHBPA. Metabolism of tHBPA to salicylaldehyde by hydration and aldol cleavage is catalyzed by a single enzyme encoded by a 1-kb MluI-StuI restriction fragment. A mechanism for the hydratase-aldolase-catalyzed reaction is proposed. The salicylaldehyde dehydrogenase gene, nahF, was cloned on a 2.75-kb BamHI fragment which also carries the naphthalene dihydrodiol dehydrogenase gene, nahB. On the basis of the identification of the enzymes encoded by various clones, the gene order for the nah operon was shown to be p, A, B, F, C, E, D.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. A rapid procedure for DNA sequencing using transposon-promoted deletions in Escherichia coli. Gene. 1985;39(2-3):305–310. doi: 10.1016/0378-1119(85)90328-2. [DOI] [PubMed] [Google Scholar]
- Ahmed A. Use of transposon-promoted deletions in DNA sequence analysis. Methods Enzymol. 1987;155:177–204. doi: 10.1016/0076-6879(87)55016-9. [DOI] [PubMed] [Google Scholar]
- Barnsley E. A. Naphthalene metabolism by pseudomonads: the oxidation of 1,2-dihydroxynaphthalene to 2-hydroxychromene-2-carboxylic acid and the formation of 2'-hydroxybenzalpyruvate. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1116–1121. doi: 10.1016/s0006-291x(76)80247-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Catterall F. A., Murray K., Williams P. A. The configuration of the 1,2-dihydroxy-1,2-dihydronaphthalene formed in the bacterial metabolism of naphthalene. Biochim Biophys Acta. 1971 May 18;237(2):361–364. doi: 10.1016/0304-4165(71)90331-x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. I., Evans W. C. Oxidative metabolism of naphthalene by soil pseudomonads. The ring-fission mechanism. Biochem J. 1964 May;91(2):251–261. doi: 10.1042/bj0910251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- EVANS W. C., FERNLEY H. N., GRIFFITHS E. OXIDATIVE METABOLISM OF PHENANTHRENE AND ANTHRACENE BY SOIL PSEUDOMONADS. THE RING-FISSION MECHANISM. Biochem J. 1965 Jun;95:819–831. doi: 10.1042/bj0950819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Karns J. S. Cloning and analysis of s-triazine catabolic genes from Pseudomonas sp. strain NRRLB-12227. J Bacteriol. 1991 Feb;173(3):1215–1222. doi: 10.1128/jb.173.3.1215-1222.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Ribbons D. W. Metabolism of dibutylphthalate and phthalate by Micrococcus sp. strain 12B. J Bacteriol. 1982 Jul;151(1):48–57. doi: 10.1128/jb.151.1.48-57.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. W., Timmis K. N. Characterization of a plasmid-specified pathway for catabolism of isopropylbenzene in Pseudomonas putida RE204. J Bacteriol. 1986 Oct;168(1):123–131. doi: 10.1128/jb.168.1.123-131.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T., Laborde A. L. Oxidation of naphthalene by a multicomponent enzyme system from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1982 Mar;149(3):948–954. doi: 10.1128/jb.149.3.948-954.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Gibson D. T. Naphthalene dioxygenase: purification and properties of a terminal oxygenase component. J Bacteriol. 1983 Aug;155(2):505–511. doi: 10.1128/jb.155.2.505-511.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Ratzkin B. J., Osslund T. D., Simon M. J., Wackett L. P., Gibson D. T. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983 Oct 14;222(4620):167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS D. R., TSAI L., STADTMAN E. R. BACTERIAL DEGRADATION OF RIBOFLAVIN. V. STOICHIOMETRY OF RIBOFLAVIN DEGRADATION TO OXAMIDE AND OTHER PRODUCTS, OXIDATION OF C14-LABELED INTERMEDIATES AND ISOLATION OF THE PSEUDOMONAD EFFECTING THESE TRANSFORMATIONS. Arch Biochem Biophys. 1964 Nov;108:323–333. doi: 10.1016/0003-9861(64)90393-5. [DOI] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of NADH-ferredoxinNAP reductase, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):457–464. doi: 10.1128/jb.172.1.457-464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler B. E., Gibson D. T. Purification and properties of ferredoxinNAP, a component of naphthalene dioxygenase from Pseudomonas sp. strain NCIB 9816. J Bacteriol. 1990 Jan;172(1):465–468. doi: 10.1128/jb.172.1.465-468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Rekik M. Bacterial aromatic ring-cleavage enzymes are classified into two different gene families. J Biol Chem. 1989 Sep 15;264(26):15328–15333. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Yeh H. J., Jerina D. M., Patel T. R., Davey J. F., Gibson D. T. Initial reactions in the oxidation of naphthalene by Pseudomonas putida. Biochemistry. 1975 Feb 11;14(3):575–584. doi: 10.1021/bi00674a018. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Kersten P. J., Dagley S., Whittaker J. W., Arciero D. M., Lipscomb J. D. 2-pyrone-4,6-dicarboxylic acid, a catabolite of gallic acids in Pseudomonas species. J Bacteriol. 1982 Dec;152(3):1154–1162. doi: 10.1128/jb.152.3.1154-1162.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhm A. E., Stolz A., Ngai K. L., Knackmuss H. J. Purification and characterization of a 1,2-dihydroxynaphthalene dioxygenase from a bacterium that degrades naphthalenesulfonic acids. J Bacteriol. 1991 Jun;173(12):3795–3802. doi: 10.1128/jb.173.12.3795-3802.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkela S., Lehväslaiho H., Palva E. T., Teeri T. H. Cloning, nucleotide sequence and characterization of genes encoding naphthalene dioxygenase of Pseudomonas putida strain NCIB9816. Gene. 1988 Dec 20;73(2):355–362. doi: 10.1016/0378-1119(88)90500-8. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K. Isolation and identification of the reaction product of alpha-hydroxy-gamma-carboxymuconic epsilon-semialdehyde dehydrogenase. J Biochem. 1979 Dec;86(6):1671–1677. doi: 10.1093/oxfordjournals.jbchem.a132687. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Purification and properties of 2-pyrone-4,6-dicarboxylate hydrolase. J Biochem. 1983 Feb;93(2):557–565. doi: 10.1093/oxfordjournals.jbchem.a134210. [DOI] [PubMed] [Google Scholar]
- Morales V. M., Bäckman A., Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991 Jan 2;97(1):39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- O'Connor C. D., Humphreys G. O. Expression of the Eco RI restriction-modification system and the construction of positive-selection cloning vectors. Gene. 1982 Dec;20(2):219–229. doi: 10.1016/0378-1119(82)90041-5. [DOI] [PubMed] [Google Scholar]
- Patel T. R., Barnsley E. A. Naphthalene metabolism by pseudomonads: purification and properties of 1,2-dihydroxynaphthalene oxygenase. J Bacteriol. 1980 Aug;143(2):668–673. doi: 10.1128/jb.143.2.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z. G., Wu R. A simple and rapid nucleotide sequencing strategy and its application in analyzing a rice histone 3 gene. Gene. 1986;45(3):247–252. doi: 10.1016/0378-1119(86)90022-3. [DOI] [PubMed] [Google Scholar]
- Royle P. L., Matsumoto H., Holloway B. W. Genetic circularity of the Pseudomonas aeruginosa PAO chromosome. J Bacteriol. 1981 Jan;145(1):145–155. doi: 10.1128/jb.145.1.145-155.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell M. A. Cloning and expression in Escherichia coli of the naphthalene degradation genes from plasmid NAH7. J Bacteriol. 1983 Feb;153(2):822–829. doi: 10.1128/jb.153.2.822-829.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yen K. M., Gunsalus I. C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci U S A. 1982 Feb;79(3):874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K. M., Serdar C. M. Genetics of naphthalene catabolism in pseudomonads. Crit Rev Microbiol. 1988;15(3):247–268. doi: 10.3109/10408418809104459. [DOI] [PubMed] [Google Scholar]


