Abstract
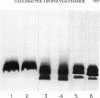
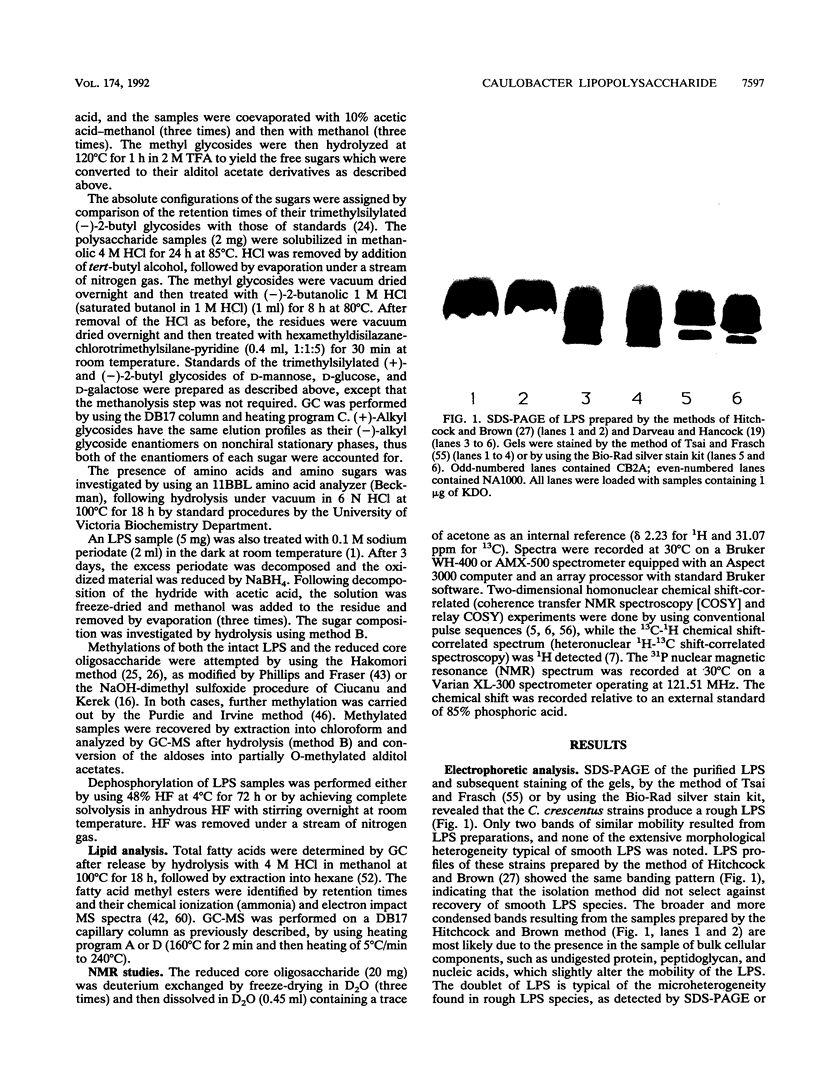
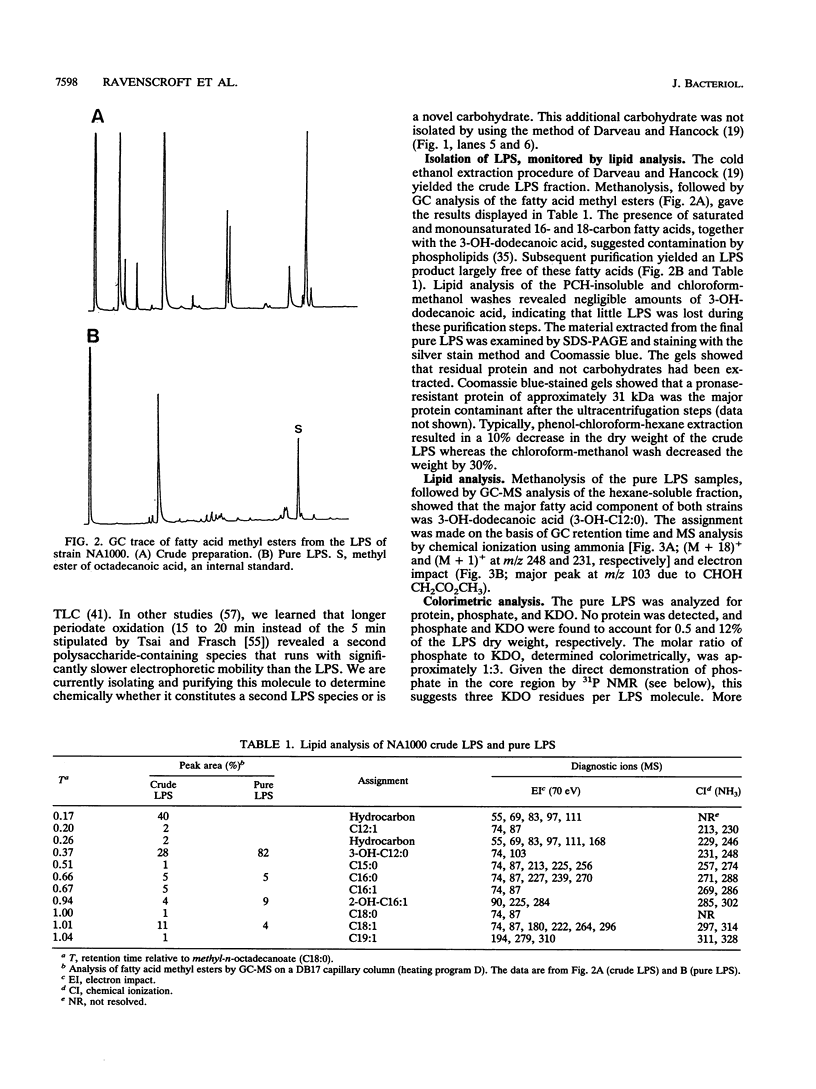
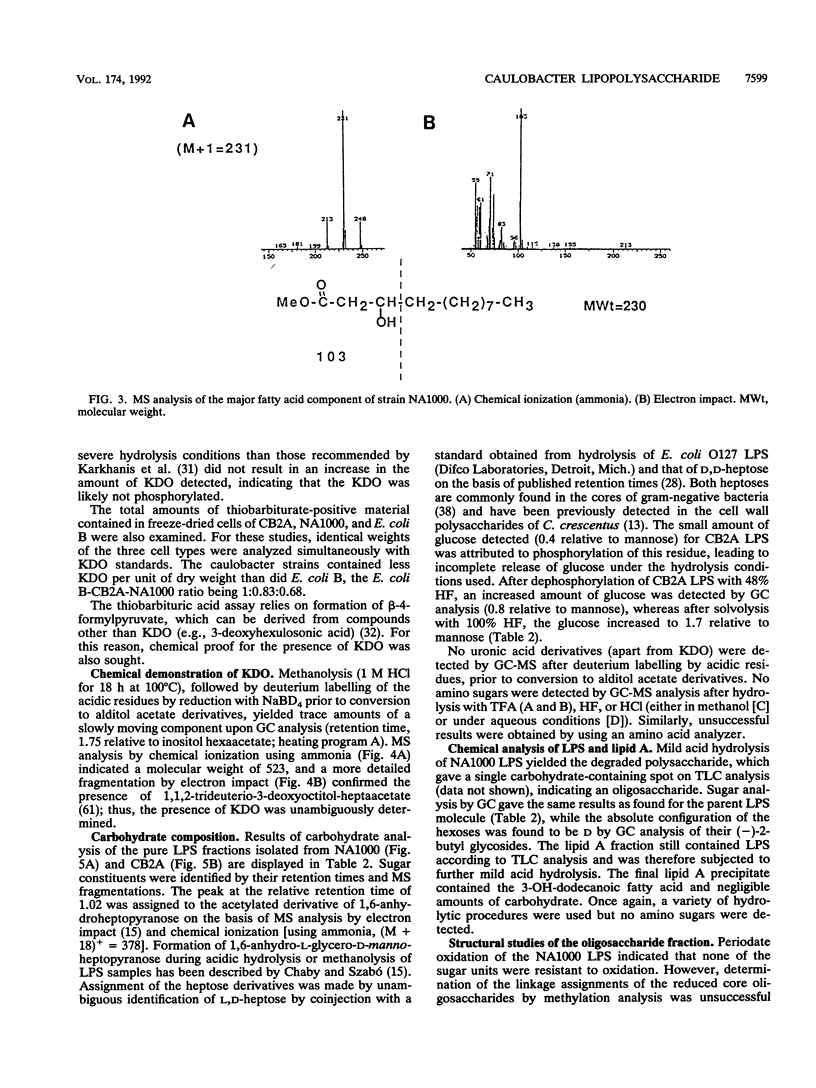
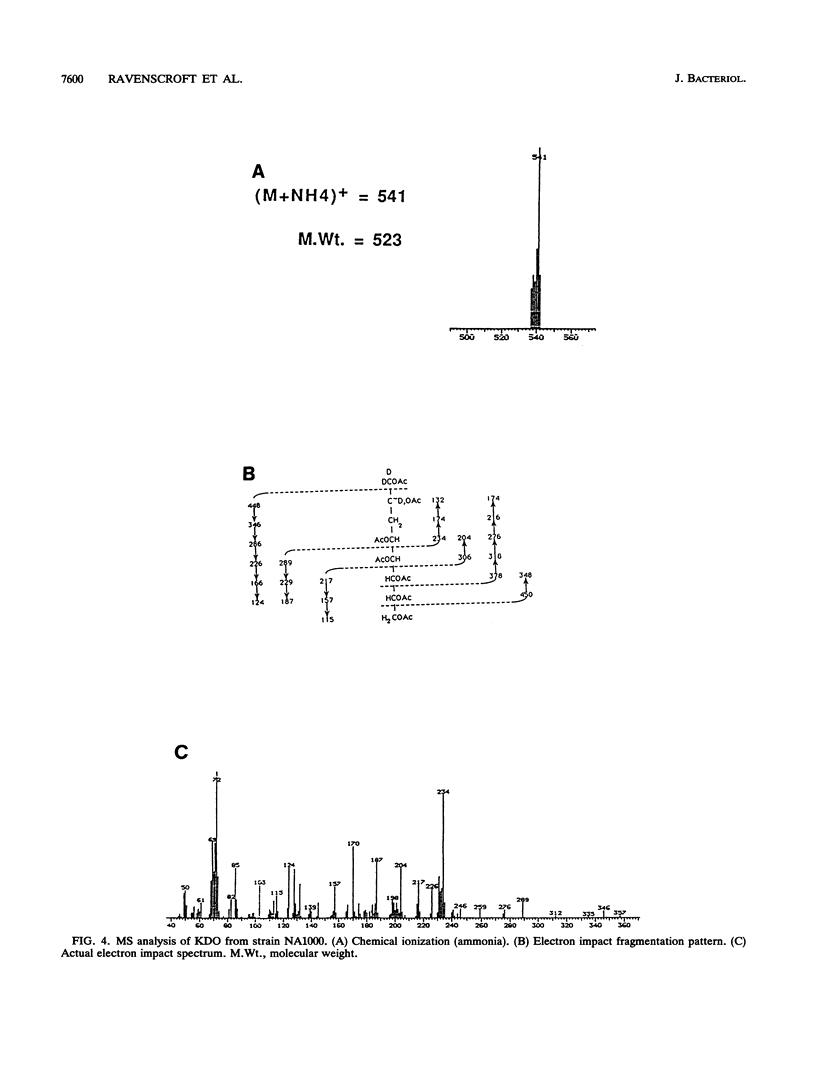
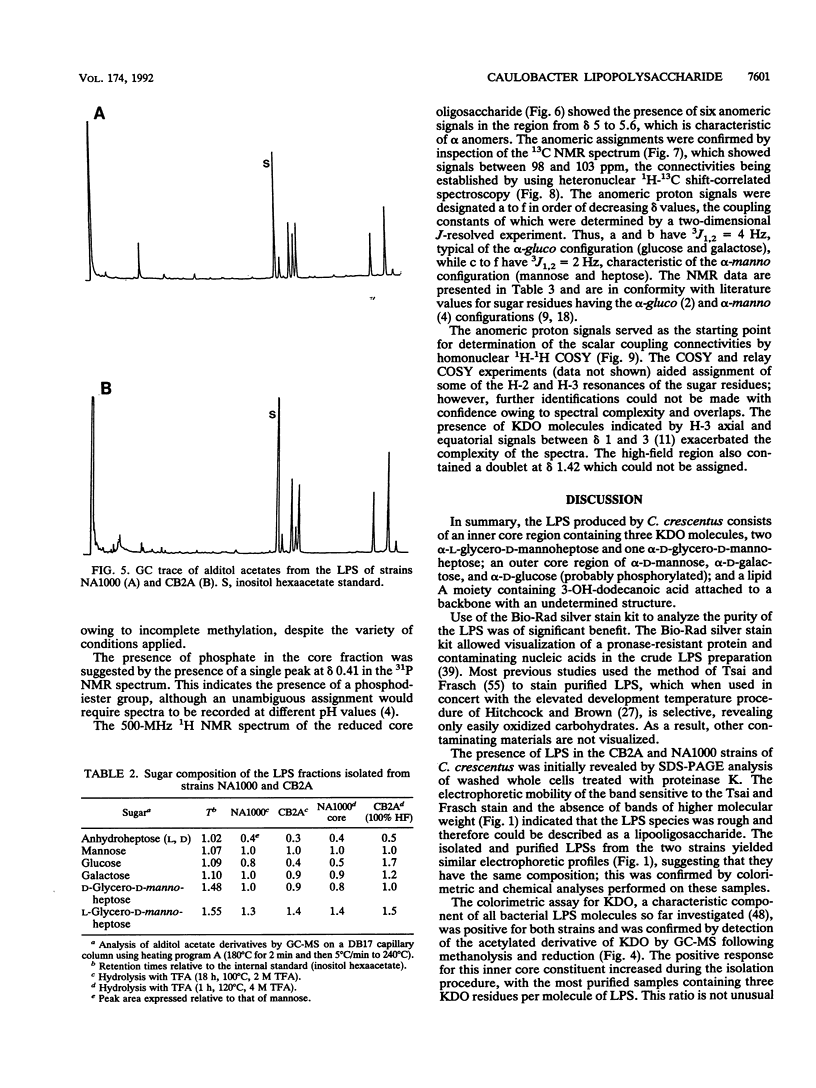
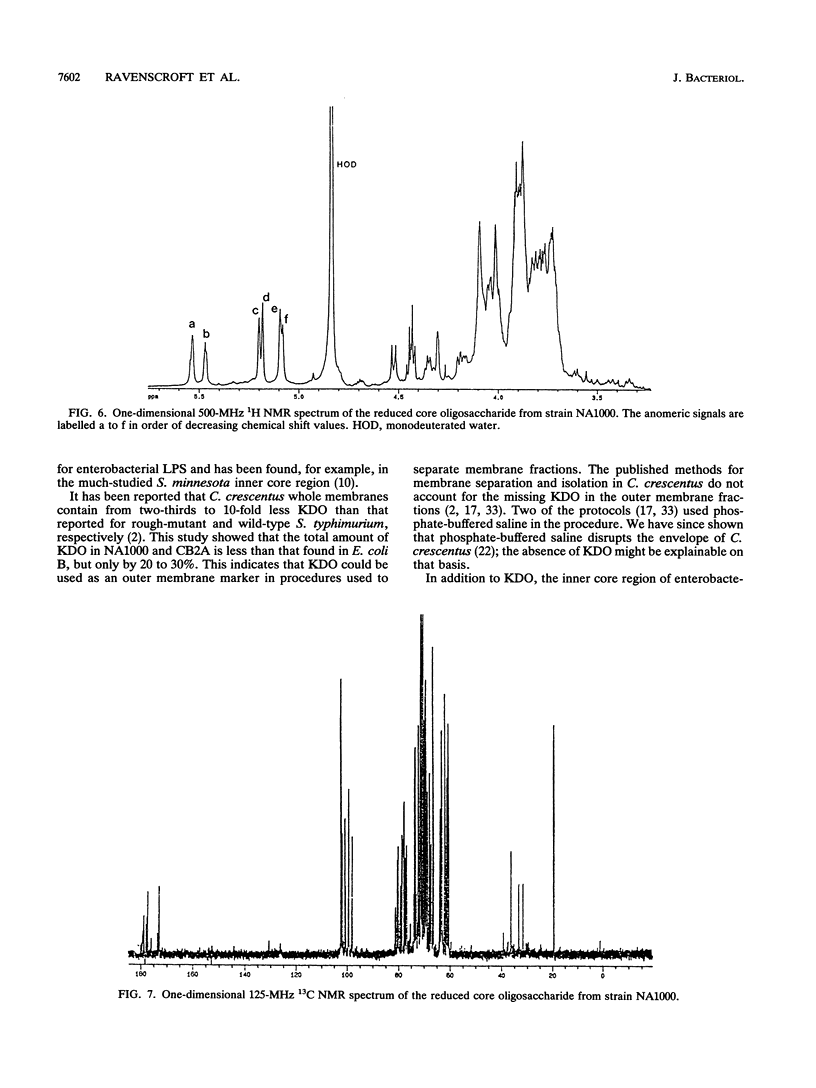
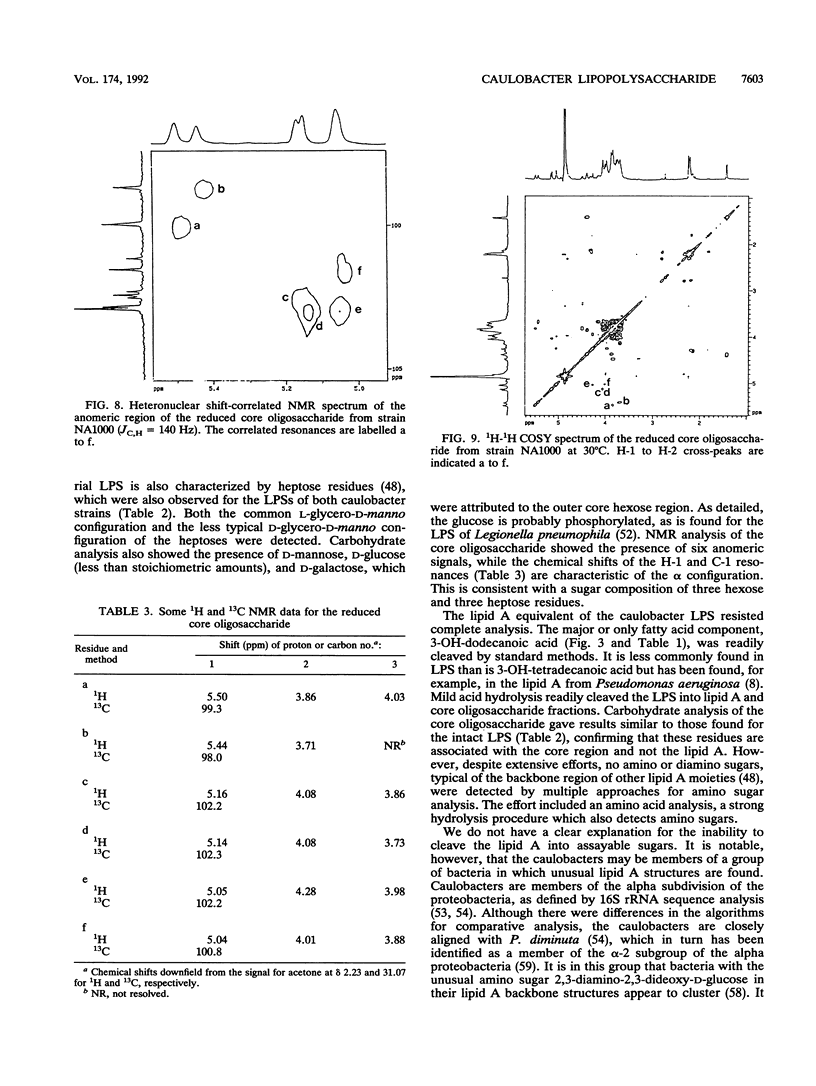
The lipopolysaccharide (LPS) of the outer membrane of Caulobacter crescentus was purified and analyzed. Two distinct strains of the species, NA 1000 and CB2A, were examined; despite differences in other membrane-related polysaccharides, the two gave similar LPS composition profiles. The LPS was the equivalent of the rough LPS described for other bacteria in that it lacked the ladder of polysaccharide-containing species that results from addition of variable amounts of a repeated sequence of sugars, as detected by gel electrophoresis in smooth LPS strains. The purified LPS contained two definable regions: (i) an oligosaccharide region, consisting of an inner core of three residues of 2-keto-3-deoxyoctonate, two residues of alpha-L-glycero-D-mannoheptose, and one alpha-D-glycero-D-mannoheptose unit and an outer core region containing one residue each of alpha-D-mannose, alpha-D-galactose, and alpha-D-glucose, with the glucose likely phosphorylated and (ii) a region equivalent to the lipid A region of the archetype, consisting primarily of an esterified fatty acid, 3-OH-dodecanoate. The lipid A-like region was resistant to conclusive analysis; in particular, although a variety of analytical methods were used, no amino sugars were detected, as is found in the lipid A of the LPS of most bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Agabian N., Unger B. Caulobacter crescentus cell envelope: effect of growth conditions on murein and outer membrane protein composition. J Bacteriol. 1978 Feb;133(2):987–994. doi: 10.1128/jb.133.2.987-994.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R., Marx A., Galanos C., Conrad R. S. Structural studies of lipid A from Pseudomonas aeruginosa PAO1: occurrence of 4-amino-4-deoxyarabinose. J Bacteriol. 1990 Dec;172(12):6631–6636. doi: 10.1128/jb.172.12.6631-6636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H., Brade L., Rietschel E. T. Structure-activity relationships of bacterial lipopolysaccharides (endotoxins). Current and future aspects. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):151–179. doi: 10.1016/s0176-6724(88)80001-4. [DOI] [PubMed] [Google Scholar]
- Brade L., Brade H. Characterization of two different antibody specificities recognizing distinct antigenic determinants in free lipid A of Escherichia coli. Infect Immun. 1985 Jun;48(3):776–781. doi: 10.1128/iai.48.3.776-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff M. G., Karibian D. Several uses for isobutyric Acid-ammonium hydroxide solvent in endotoxin analysis. Appl Environ Microbiol. 1990 Jun;56(6):1957–1959. doi: 10.1128/aem.56.6.1957-1959.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaby R., Szabó P. Fromation of 1,6-anhydro-L-glycero-D-manno-heptopyranose during acidic hydrolysis and methanolysis of a polysaccharide from Bordetella pertussis. Carbohydr Res. 1976 Jul;49:489–493. doi: 10.1016/s0008-6215(00)83167-5. [DOI] [PubMed] [Google Scholar]
- Clancy M. J., Newton A. Localization of proteins in the inner and outer membranes of Caulobacter crescentus. Biochim Biophys Acta. 1982 Apr 7;686(2):160–169. doi: 10.1016/0005-2736(82)90108-0. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P., Smit J. A transducing bacteriophage for Caulobacter crescentus uses the paracrystalline surface layer protein as a receptor. J Bacteriol. 1991 Sep;173(17):5568–5572. doi: 10.1128/jb.173.17.5568-5572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Harris P. J., Henry R. J., Blakeney A. B., Stone B. A. An improved procedure for the methylation analysis of oligosaccharides and polysaccharides. Carbohydr Res. 1984 Apr 2;127(1):59–73. doi: 10.1016/0008-6215(84)85106-x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst O., Brade H., Dziewiszek K., Zamojski A. G.l.c.-m.s. of partially methylated and acetylated derivatives of L-glycero-D-manno- and D-glycero-D-manno-heptopyranoses and -heptitols. Carbohydr Res. 1990 Sep 5;204:1–9. doi: 10.1016/0008-6215(90)84016-n. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kondo S., Zähringer U., Rietschel E. T., Hisatsune K. Isolation and identification of 3-deoxy-D-threo-hexulosonic acid as a constituent of the lipopolysaccharide of Vibrio parahaemolyticus serotypes O7 and O12. Carbohydr Res. 1989 Jun 1;188:97–104. doi: 10.1016/0008-6215(89)84062-5. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Fukuda A., Okada Y. The penicillin-binding proteins of Caulobacter crescentus. J Biochem. 1980 Jan;87(1):363–366. doi: 10.1093/oxfordjournals.jbchem.a132749. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letts V., Shaw P., Shapiro L., Henry S. Synthesis and utilization of fatty acids by wild-type and fatty acid auxotrophs of Caulobacter crescentus. J Bacteriol. 1982 Sep;151(3):1269–1278. doi: 10.1128/jb.151.3.1269-1278.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981 Mar;45(1):123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenscroft N., Walker S. G., Dutton G. G., Smit J. Identification, isolation, and structural studies of extracellular polysaccharides produced by Caulobacter crescentus. J Bacteriol. 1991 Sep;173(18):5677–5684. doi: 10.1128/jb.173.18.5677-5684.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cell surface patterning and morphogenesis: biogenesis of a periodic surface array during Caulobacter development. J Cell Biol. 1982 Oct;95(1):41–49. doi: 10.1083/jcb.95.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cloning of the major protein of the Caulobacter crescentus periodic surface layer: detection and characterization of the cloned peptide by protein expression assays. J Bacteriol. 1984 Dec;160(3):1137–1145. doi: 10.1128/jb.160.3.1137-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonesson A., Jantzen E., Bryn K., Larsson L., Eng J. Chemical composition of a lipopolysaccharide from Legionella pneumophila. Arch Microbiol. 1989;153(1):72–78. doi: 10.1007/BF00277544. [DOI] [PubMed] [Google Scholar]
- Stackebrandt E., Fischer A., Roggentin T., Wehmeyer U., Bomar D., Smida J. A phylogenetic survey of budding, and/or prosthecate, non-phototrophic eubacteria: membership of Hyphomicrobium, Hyphomonas, Pedomicrobium, Filomicrobium, Caulobacter and "dichotomicrobium" to the alpha-subdivision of purple non-sulfur bacteria. Arch Microbiol. 1988;149(6):547–556. doi: 10.1007/BF00446759. [DOI] [PubMed] [Google Scholar]
- Stahl D. A., Key R., Flesher B., Smit J. The phylogeny of marine and freshwater caulobacters reflects their habitat. J Bacteriol. 1992 Apr;174(7):2193–2198. doi: 10.1128/jb.174.7.2193-2198.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Walker S. G., Smith S. H., Smit J. Isolation and comparison of the paracrystalline surface layer proteins of freshwater caulobacters. J Bacteriol. 1992 Mar;174(6):1783–1792. doi: 10.1128/jb.174.6.1783-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckesser J., Mayer H. Different lipid A types in lipopolysaccharides of phototrophic and related non-phototrophic bacteria. FEMS Microbiol Rev. 1988 Apr-Jun;4(2):143–153. doi: 10.1111/j.1574-6968.1988.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]