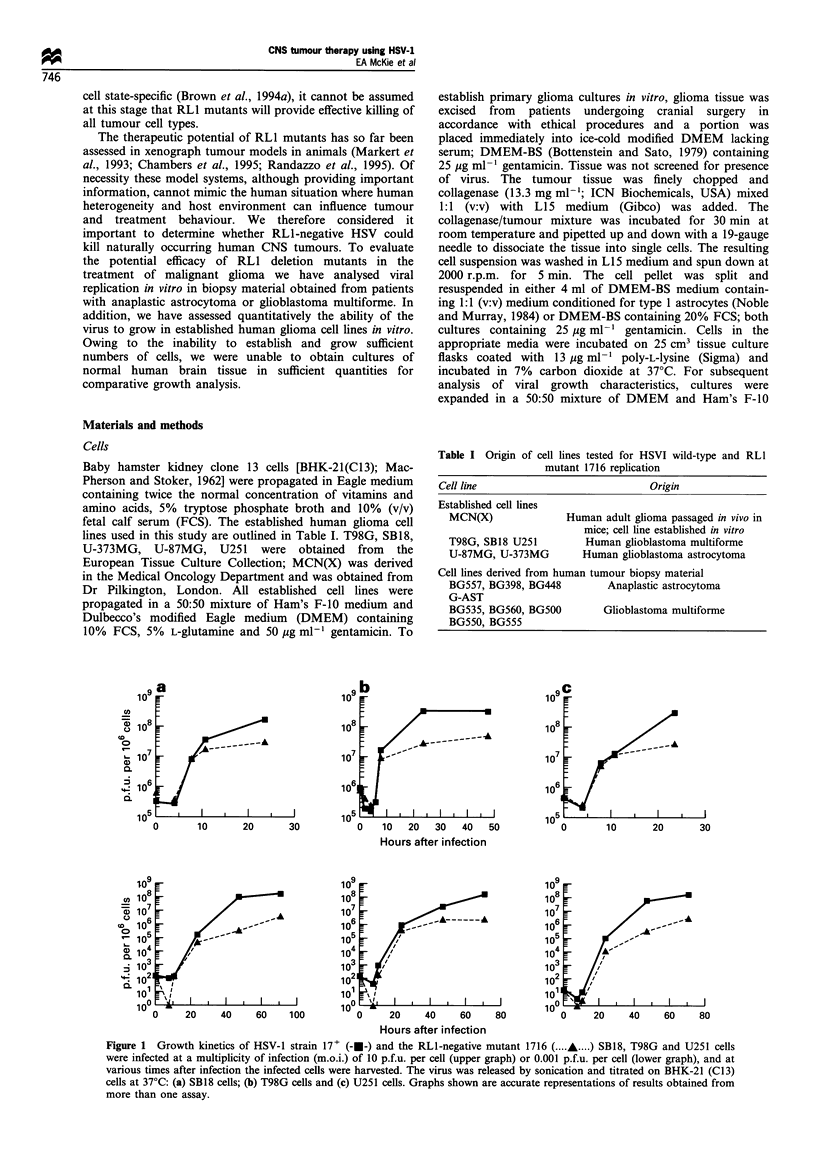
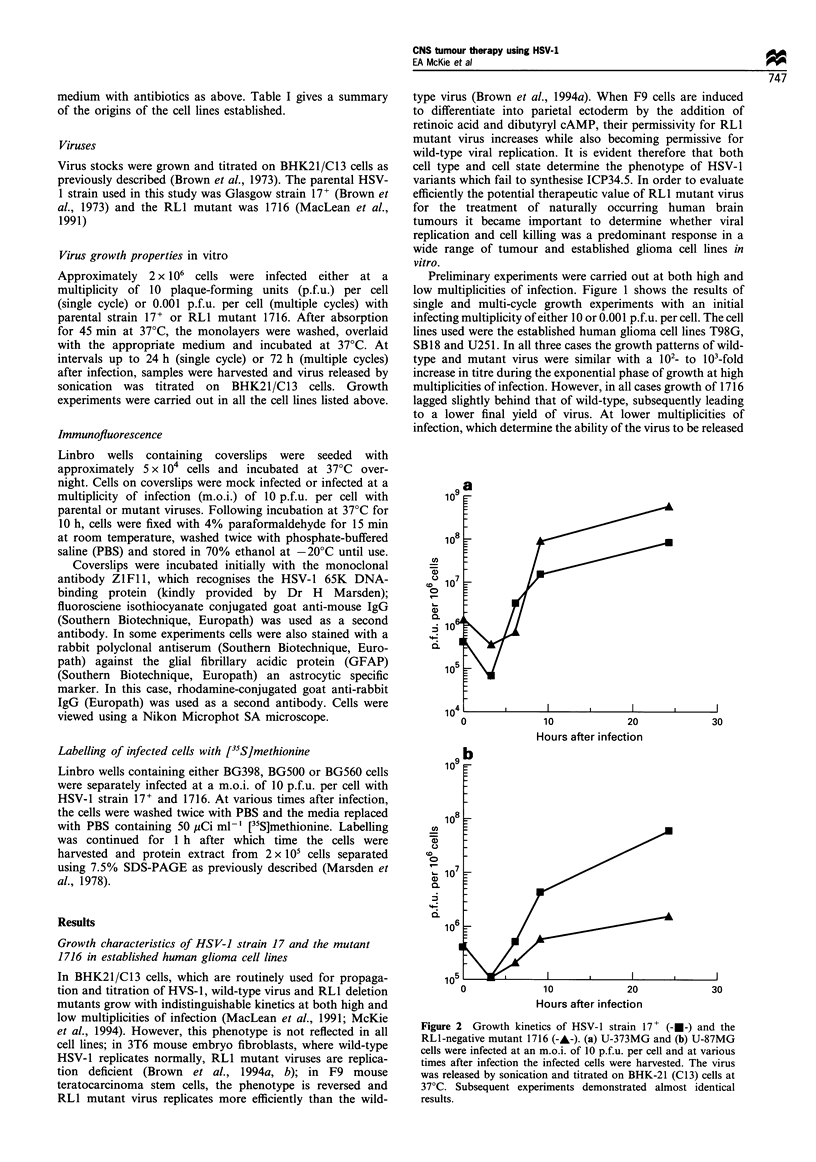
Abstract
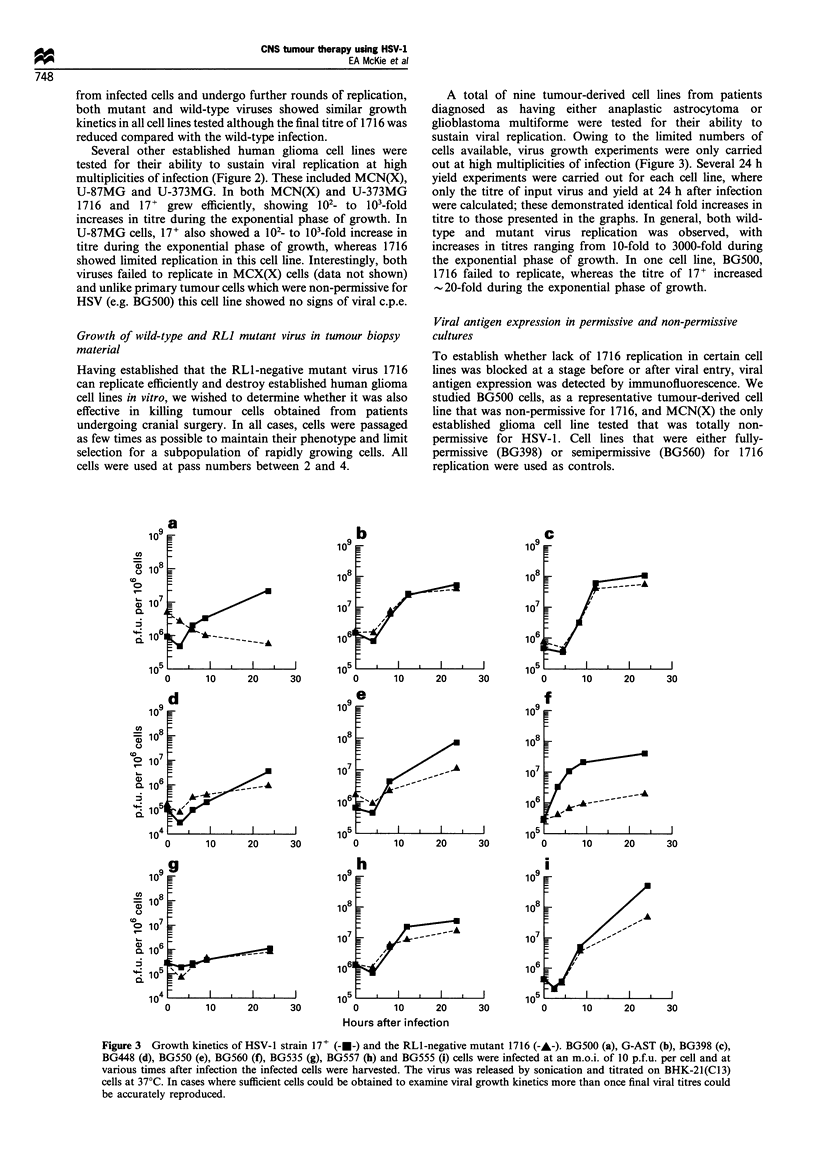
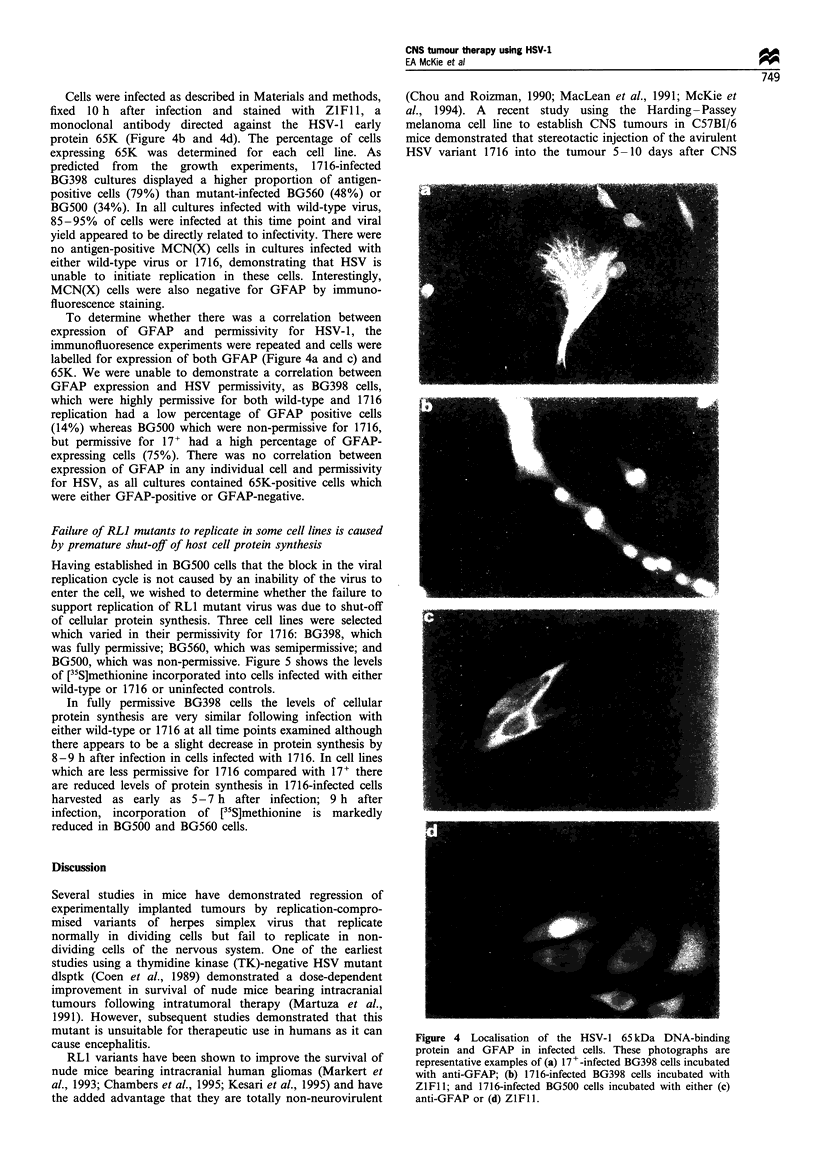
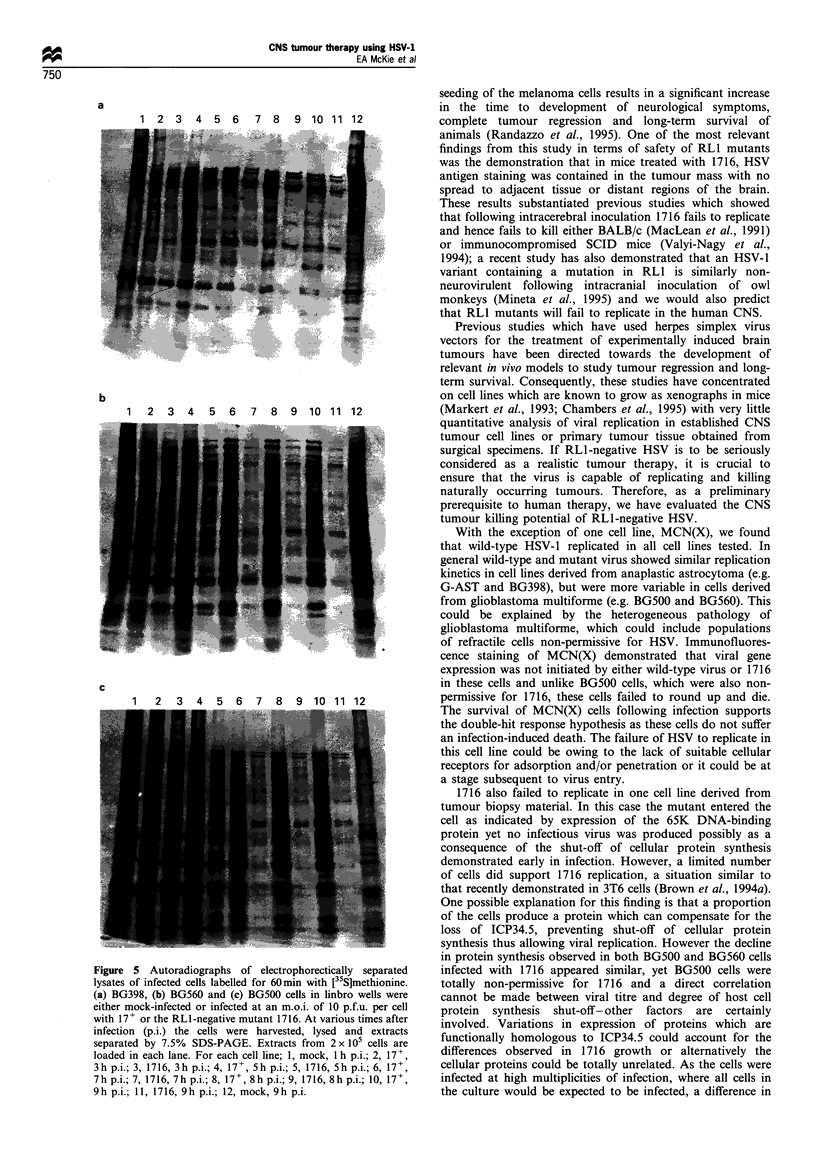
Primary tumours of the central nervous system (CNS) are an important cause of cancer-related deaths in adults and children. CNS tumours are mostly glial cell in origin and are predominantly astrocytomas. Conventional therapy of high-grade gliomas includes maximal resection followed by radiation treatment. The addition of adjuvant chemotherapy provides little improvement in survival time and hence assessment of novel therapies is imperative. We have evaluated the potential therapeutic use of the herpes simplex virus (HSV) mutant 1716 in the treatment of primary brain tumours. The mutant is deleted in the RL1 gene and fails to produce the virulence factor ICP34.5. 1716 replication was analysed in both established human glioma cell lines and in primary cell cultures derived from human tumour biopsy material. In the majority of cultures, virus replication occurred and consequential cell death resulted. In the minority of tumour cell lines which are non-permissive for mutant replication, premature shut-off of host cell protein synthesis was induced in response to lack of expression of ICP34.5. Hence RL1-negative mutants have the distinct advantage of providing a double hit phenomenon whereby cell death could occur by either pathway. Moreover, 1716, by virtue of its ability to replicate selectively within a tumour cell, has the potential to deliver a 'suicide' gene product to the required site immediately. It is our opinion that HSV which fails to express ICP34.5 could provide an effective tumour therapy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allalunis-Turner M. J., Barron G. M., Day R. S., 3rd, Dobler K., Urtasun R. C. Heterogeneity in response to treatment with buthionine sulfoximine or interferon in human malignant glioma cells. Int J Radiat Oncol Biol Phys. 1992;22(4):765–768. doi: 10.1016/0360-3016(92)90520-r. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Bullard D. E., Mahaley M. S., Jr, Bigner D. D. Chromosomal evolution in malignant human gliomas starts with specific and usually numerical deviations. Cancer Genet Cytogenet. 1986 Jun;22(2):121–135. doi: 10.1016/0165-4608(86)90172-x. [DOI] [PubMed] [Google Scholar]
- Bigner S. H., Mark J., Mahaley M. S., Bigner D. D. Patterns of the early, gross chromosomal changes in malignant human gliomas. Hereditas. 1984;101(1):103–113. doi: 10.1111/j.1601-5223.1984.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Harland J., MacLean A. R., Podlech J., Clements J. B. Cell type and cell state determine differential in vitro growth of non-neurovirulent ICP34.5-negative herpes simplex virus types 1 and 2. J Gen Virol. 1994 Sep;75(Pt 9):2367–2377. doi: 10.1099/0022-1317-75-9-2367. [DOI] [PubMed] [Google Scholar]
- Brown S. M., MacLean A. R., Aitken J. D., Harland J. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J Gen Virol. 1994 Dec;75(Pt 12):3679–3686. doi: 10.1099/0022-1317-75-12-3679. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Chambers R., Gillespie G. Y., Soroceanu L., Andreansky S., Chatterjee S., Chou J., Roizman B., Whitley R. J. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Horton J., Schoenfeld D., Salazer O., Perez-Tamayo R., Kramer S., Weinstein A., Nelson J. S., Tsukada Y. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983 Sep 15;52(6):997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Chou J., Kern E. R., Whitley R. J., Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990 Nov 30;250(4985):1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Chou J., Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J., Roizman B. The terminal a sequence of the herpes simplex virus genome contains the promoter of a gene located in the repeat sequences of the L component. J Virol. 1986 Feb;57(2):629–637. doi: 10.1128/jvi.57.2.629-637.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Kosz-Vnenchak M., Jacobson J. G., Leib D. A., Bogard C. L., Schaffer P. A., Tyler K. L., Knipe D. M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan A., McKie E., MacLean A. R., McGeoch D. J. Status of the ICP34.5 gene in herpes simplex virus type 1 strain 17. J Gen Virol. 1992 Apr;73(Pt 4):971–973. doi: 10.1099/0022-1317-73-4-971. [DOI] [PubMed] [Google Scholar]
- Fine H. A., Dear K. B., Loeffler J. S., Black P. M., Canellos G. P. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993 Apr 15;71(8):2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kelly P. J., Daumas-Duport C., Kispert D. B., Kall B. A., Scheithauer B. W., Illig J. J. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987 Jun;66(6):865–874. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- Kesari S., Randazzo B. P., Valyi-Nagy T., Huang Q. S., Brown S. M., MacLean A. R., Lee V. M., Trojanowski J. Q., Fraser N. W. Therapy of experimental human brain tumors using a neuroattenuated herpes simplex virus mutant. Lab Invest. 1995 Nov;73(5):636–648. [PubMed] [Google Scholar]
- Kornblith P. L., Walker M. Chemotherapy for malignant gliomas. J Neurosurg. 1988 Jan;68(1):1–17. doi: 10.3171/jns.1988.68.1.0001. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., STOKER M. Polyoma transformation of hamster cell clones--an investigation of genetic factors affecting cell competence. Virology. 1962 Feb;16:147–151. doi: 10.1016/0042-6822(62)90290-8. [DOI] [PubMed] [Google Scholar]
- MacLean A. R., ul-Fareed M., Robertson L., Harland J., Brown S. M. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the 'a' sequence. J Gen Virol. 1991 Mar;72(Pt 3):631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- Markert J. M., Malick A., Coen D. M., Martuza R. L. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993 Apr;32(4):597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Stow N. D., Preston V. G., Timbury M. C., Wilkie N. M. Physical mapping of herpes simplex virus-induced polypeptides. J Virol. 1978 Nov;28(2):624–642. doi: 10.1128/jvi.28.2.624-642.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuza R. L., Malick A., Markert J. M., Ruffner K. L., Coen D. M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991 May 10;252(5007):854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- McKie E. A., Hope R. G., Brown S. M., MacLean A. R. Characterization of the herpes simplex virus type 1 strain 17+ neurovirulence gene RL1 and its expression in a bacterial system. J Gen Virol. 1994 Apr;75(Pt 4):733–741. doi: 10.1099/0022-1317-75-4-733. [DOI] [PubMed] [Google Scholar]
- Mehta M. P., Masciopinto J., Rozental J., Levin A., Chappell R., Bastin K., Miles J., Turski P., Kubsad S., Mackie T. Stereotactic radiosurgery for glioblastoma multiforme: report of a prospective study evaluating prognostic factors and analyzing long-term survival advantage. Int J Radiat Oncol Biol Phys. 1994 Oct 15;30(3):541–549. doi: 10.1016/0360-3016(92)90939-f. [DOI] [PubMed] [Google Scholar]
- Mineta T., Rabkin S. D., Martuza R. L. Treatment of malignant gliomas using ganciclovir-hypersensitive, ribonucleotide reductase-deficient herpes simplex viral mutant. Cancer Res. 1994 Aug 1;54(15):3963–3966. [PubMed] [Google Scholar]
- Mineta T., Rabkin S. D., Yazaki T., Hunter W. D., Martuza R. L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995 Sep;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Noble M., Murray K. Purified astrocytes promote the in vitro division of a bipotential glial progenitor cell. EMBO J. 1984 Oct;3(10):2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E. H., Ram Z., Culver K. W., Blaese R. M., DeVroom H. L., Anderson W. F. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- Rampling R., Cruickshank G., Lewis A. D., Fitzsimmons S. A., Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys. 1994 Jun 15;29(3):427–431. doi: 10.1016/0360-3016(94)90432-4. [DOI] [PubMed] [Google Scholar]
- Randazzo B. P., Kesari S., Gesser R. M., Alsop D., Ford J. C., Brown S. M., Maclean A., Fraser N. W. Treatment of experimental intracranial murine melanoma with a neuroattenuated herpes simplex virus 1 mutant. Virology. 1995 Aug 1;211(1):94–101. doi: 10.1006/viro.1995.1382. [DOI] [PubMed] [Google Scholar]
- Valyi-Nagy T., Fareed M. U., O'Keefe J. S., Gesser R. M., MacLean A. R., Brown S. M., Spivack J. G., Fraser N. W. The herpes simplex virus type 1 strain 17+ gamma 34.5 deletion mutant 1716 is avirulent in SCID mice. J Gen Virol. 1994 Aug;75(Pt 8):2059–2063. doi: 10.1099/0022-1317-75-8-2059. [DOI] [PubMed] [Google Scholar]