Abstract
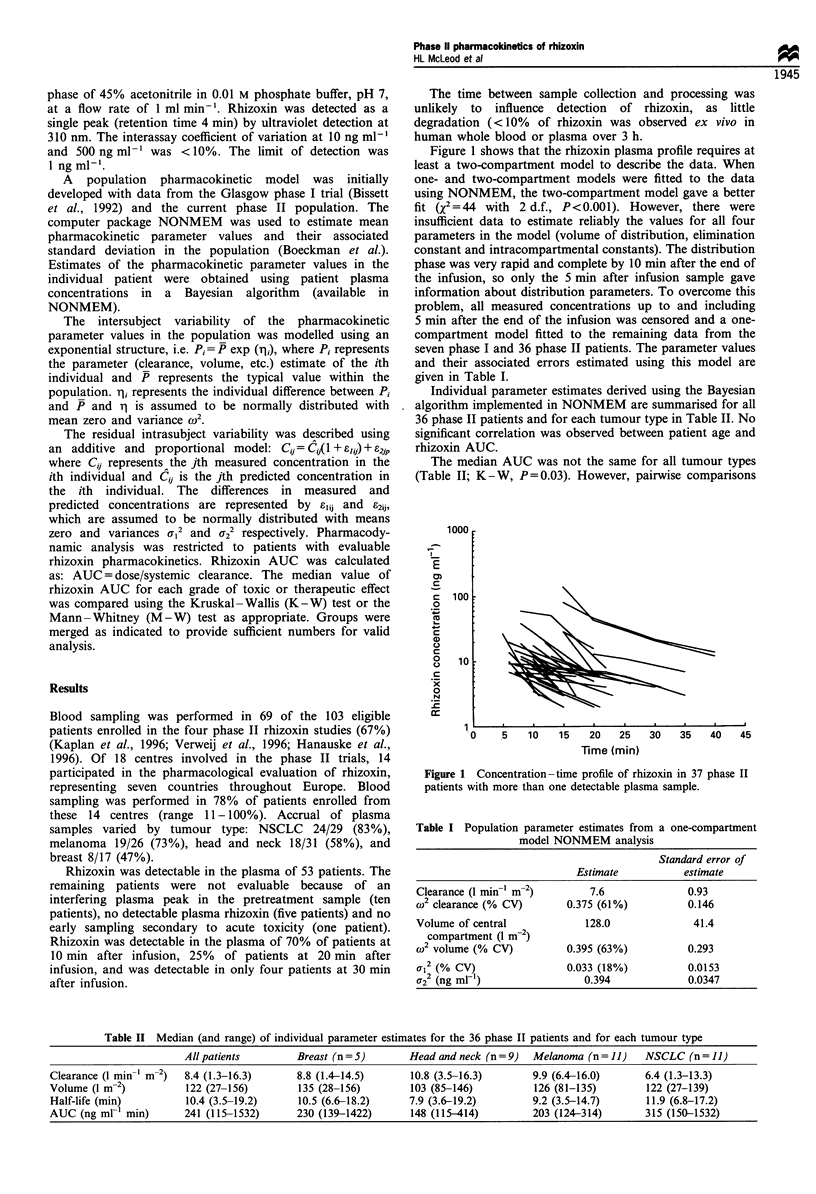
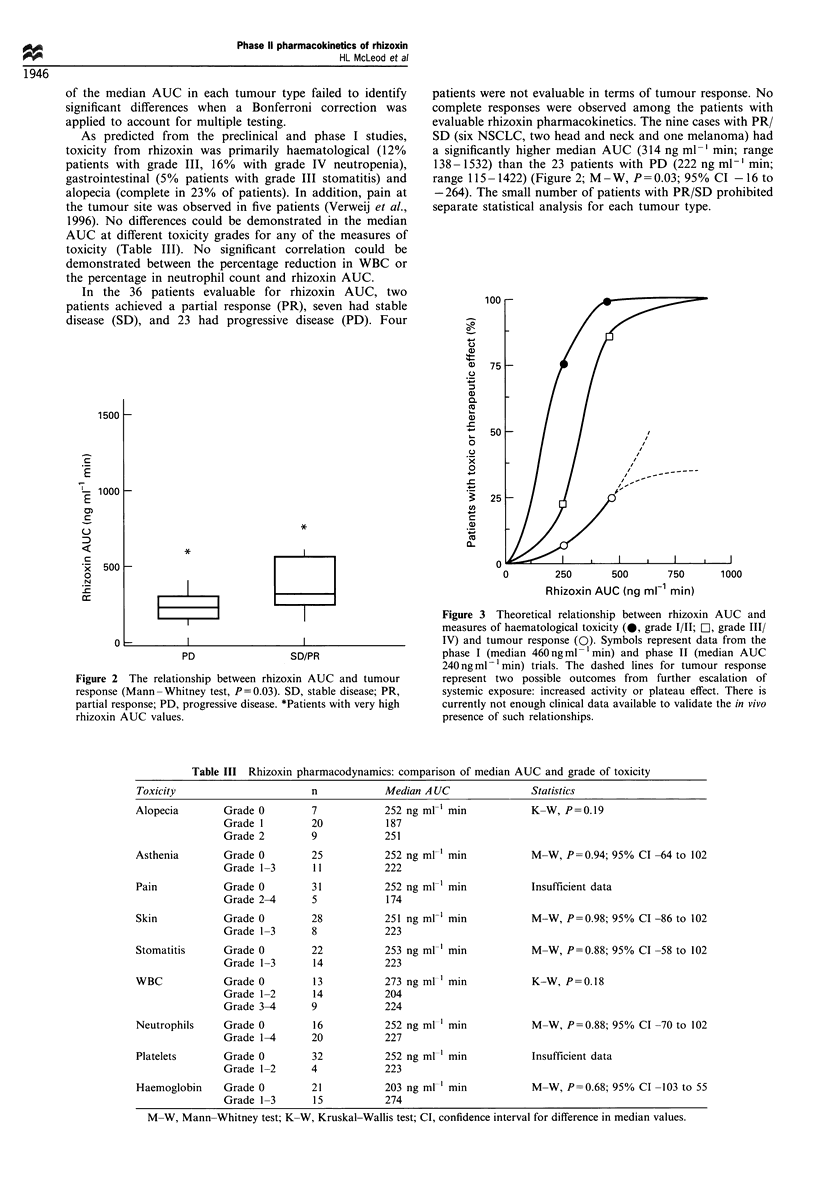
Rhizoxin is a macrocyclic lactone compound that binds to tubulin and inhibits microtubule assembly. Rhizoxin demonstrated preclinical anti-tumour activity against a variety of human tumour cell lines and xenograft models. Phase I evaluation found a maximum tolerated rhizoxin dose of 2.6 mg m-2, with reversible, but dose-limiting, mucositis, leucopenia and diarrhoea. Clinical trials were then initiated by the EORTC ECSG in melanoma, breast, head and neck, and non-small-cell lung cancers with the recommended phase II rhizoxin dose of 2 mg m-2. Pharmacological studies were instituted with the phase II trials to complement the limited pharmacokinetic data available from the phase I trial. Blood samples were obtained from 69 of 103 eligible patients enrolled in phase II rhizoxin studies, and these were evaluable for pharmacokinetic analysis in 36 patients. Plasma rhizoxin concentrations were determined by high-performance liquid chromatography (HPLC), and post-distribution pharmacokinetic parameters were estimated by a one-compartment model. Rhizoxin was rapidly eliminated from plasma, with a median systemic clearance of 8.41 min-1 m-2 and an elimination half-life of 10.4 min. Rhizoxin area under the concentration-time curve (AUC) was higher in patients obtaining a partial response or stable disease than in those with progressive disease (median 314 vs 222 ng ml-1 min; P = 0.03). As predicted from previous studies, haematological and gastrointestinal toxicity was observed, but could not be shown to be related to rhizoxin AUC. This study demonstrated the rapid and variable elimination of rhizoxin from the systemic circulation. The presence of pharmacodynamic relationships and the low level of systemic toxicity suggest that future trials of rhizoxin with alternative dosage or treatment schedules are warranted.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissett D., Graham M. A., Setanoians A., Chadwick G. A., Wilson P., Koier I., Henrar R., Schwartsmann G., Cassidy J., Kaye S. B. Phase I and pharmacokinetic study of rhizoxin. Cancer Res. 1992 May 15;52(10):2894–2898. [PubMed] [Google Scholar]
- Gianni L., Kearns C. M., Giani A., Capri G., Viganó L., Lacatelli A., Bonadonna G., Egorin M. J. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol. 1995 Jan;13(1):180–190. doi: 10.1200/JCO.1995.13.1.180. [DOI] [PubMed] [Google Scholar]
- Graham M. A., Bissett D., Setanoians A., Hamilton T., Kerr D. J., Henrar R., Kaye S. B. Preclinical and phase I studies with rhizoxin to apply a pharmacokinetically guided dose-escalation scheme. J Natl Cancer Inst. 1992 Apr 1;84(7):494–500. doi: 10.1093/jnci/84.7.494. [DOI] [PubMed] [Google Scholar]
- Hanauske A. R., Catimel G., Aamdal S., ten Bokkel Huinink W., Paridaens R., Pavlidis N., Kaye S. B., te Velde A., Wanders J., Verweij J. Phase II clinical trials with rhizoxin in breast cancer and melanoma. The EORTC Early Clinical Trials Group. Br J Cancer. 1996 Feb;73(3):397–399. doi: 10.1038/bjc.1996.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks H. R., Plowman J., Berger D. P., Paull K. D., Fiebig H. H., Fodstad O., Dreef-van der Meulen H. C., Henrar R. E., Pinedo H. M., Schwartsmann G. Preclinical antitumour activity and animal toxicology studies of rhizoxin, a novel tubulin-interacting agent. Ann Oncol. 1992 Nov;3(9):755–763. doi: 10.1093/oxfordjournals.annonc.a058334. [DOI] [PubMed] [Google Scholar]
- Huizing M. T., Keung A. C., Rosing H., van der Kuij V., ten Bokkel Huinink W. W., Mandjes I. M., Dubbelman A. C., Pinedo H. M., Beijnen J. H. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. J Clin Oncol. 1993 Nov;11(11):2127–2135. doi: 10.1200/JCO.1993.11.11.2127. [DOI] [PubMed] [Google Scholar]
- Kaplan S., Hanauske A. R., Pavlidis N., Bruntsch U., te Velde A., Wanders J., Heinrich B., Verweij J. Single agent activity of rhizoxin in non-small-cell lung cancer: a phase II trial of the EORTC Early Clinical Trials Group. Br J Cancer. 1996 Feb;73(3):403–405. doi: 10.1038/bjc.1996.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod H. L., Graham M. A., Aamdal S., Setanoians A., Groot Y., Lund B. Phase I pharmacokinetics and limited sampling strategies for the bioreductive alkylating drug EO9. EORTC Early Clinical Trials Group. Eur J Cancer. 1996 Aug;32A(9):1518–1522. doi: 10.1016/0959-8049(96)00120-7. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., Donehower R. C., Jones R. J., Tucker R. W. Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res. 1988 Jul 15;48(14):4093–4100. [PubMed] [Google Scholar]
- Sonnichsen D. S., Hurwitz C. A., Pratt C. B., Shuster J. J., Relling M. V. Saturable pharmacokinetics and paclitaxel pharmacodynamics in children with solid tumors. J Clin Oncol. 1994 Mar;12(3):532–538. doi: 10.1200/JCO.1994.12.3.532. [DOI] [PubMed] [Google Scholar]
- Sullivan A. S., Prasad V., Roach M. C., Takahashi M., Iwasaki S., Ludueña R. F. Interaction of rhizoxin with bovine brain tubulin. Cancer Res. 1990 Jul 15;50(14):4277–4280. [PubMed] [Google Scholar]
- Tsuruo T., Oh-hara T., Iida H., Tsukagoshi S., Sato Z., Matsuda I., Iwasaki S., Okuda S., Shimizu F., Sasagawa K. Rhizoxin, a macrocyclic lactone antibiotic, as a new antitumor agent against human and murine tumor cells and their vincristine-resistant sublines. Cancer Res. 1986 Jan;46(1):381–385. [PubMed] [Google Scholar]
- Verweij J., Wanders J., Gil T., Schöffski P., Catimel G., te Velde A., de Mulder P. H. Phase II study of rhizoxin in squamous cell head and neck cancer. The EORTC Early Clinical Trials Group. Br J Cancer. 1996 Feb;73(3):400–402. doi: 10.1038/bjc.1996.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild M. D., Walle U. K., Walle T. Extensive and saturable accumulation of paclitaxel by the human platelet. Cancer Chemother Pharmacol. 1995;36(1):41–44. doi: 10.1007/BF00685730. [DOI] [PubMed] [Google Scholar]


