Abstract
Neuroblastoma is the most common extracranial solid tumour of childhood. Amplification of the proto-oncogene, N-myc, confers a poor prognosis in neuroblastoma, while hyperdiploidy is associated with a favourable outcome. Little is known about the contribution of tumour-suppressor genes to the development or progression of neuroblastoma. We examined allelic imbalance at the locus of the tumour-suppressor gene, APC (adenomatous polyposis coli), on chromosome 5q using a polymerase chain reaction (PCR)-based assay. Nine of 24 (37.5%) informative neuroblastoma tumours showed allelic imbalance (AI) at this locus. Clinical data concerning N-myc amplification and DNA content were correlated with these results in the same patients. Allelic imbalance was found only in tumours containing a single copy of the N-myc gene and exhibiting hyperdiploidy. All nine patients with AI of chromosome 5q were alive after a median follow-up period of 46 months, while 7 of 15 (47%) of those lacking AI at this locus had died (P = 0.018). Allelic imbalance at three additional loci on chromosome 5 was demonstrated in tumours that exhibited AI at the APC locus, suggesting that endoreduplication of chromosome 5 had occurred. Fluorescent in situ hybridisation (FISH) analysis of tumour tissue from one patient exhibiting AI demonstrated two, three, four or six copies of the APC gene per cell, consistent with this hypothesis. These data suggest that allelic imbalance of chromosome 5 is involved in at least a subset of neuroblastomas and influences survival in patients with neuroblastoma.
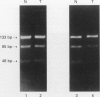
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton-Rickardt P. G., Wyllie A. H., Bird C. C., Dunlop M. G., Steel C. M., Morris R. G., Piris J., Romanowski P., Wood R., White R. MCC, a candidate familial polyposis gene in 5q.21, shows frequent allele loss in colorectal and lung cancer. Oncogene. 1991 Oct;6(10):1881–1886. [PubMed] [Google Scholar]
- Bourhis J., De Vathaire F., Wilson G. D., Hartmann O., Terrier-Lacombe M. J., Boccon-Gibod L., McNally N. J., Lemerle J., Riou G., Bénard J. Combined analysis of DNA ploidy index and N-myc genomic content in neuroblastoma. Cancer Res. 1991 Jan 1;51(1):33–36. [PubMed] [Google Scholar]
- Boynton R. F., Blount P. L., Yin J., Brown V. L., Huang Y., Tong Y., McDaniel T., Newkirk C., Resau J. H., Raskind W. H. Loss of heterozygosity involving the APC and MCC genetic loci occurs in the majority of human esophageal cancers. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3385–3388. doi: 10.1073/pnas.89.8.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Sekhon G., Goldstein M. N. Chromosomal aberrations in human neuroblastomas. Cancer. 1977 Nov;40(5):2256–2263. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Chen Y. M., Bookstein R., Lee W. H. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990 Dec 14;250(4987):1576–1580. doi: 10.1126/science.2274789. [DOI] [PubMed] [Google Scholar]
- D'Amico D., Carbone D. P., Johnson B. E., Meltzer S. J., Minna J. D. Polymorphic sites within the MCC and APC loci reveal very frequent loss of heterozygosity in human small cell lung cancer. Cancer Res. 1992 Apr 1;52(7):1996–1999. [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fong C. T., Dracopoli N. C., White P. S., Merrill P. T., Griffith R. C., Housman D. E., Brodeur G. M. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989 May;86(10):3753–3757. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C. T., White P. S., Peterson K., Sapienza C., Cavenee W. K., Kern S. E., Vogelstein B., Cantor A. B., Look A. T., Brodeur G. M. Loss of heterozygosity for chromosomes 1 or 14 defines subsets of advanced neuroblastomas. Cancer Res. 1992 Apr 1;52(7):1780–1785. [PubMed] [Google Scholar]
- Hayashi Y., Kanda N., Inaba T., Hanada R., Nagahara N., Muchi H., Yamamoto K. Cytogenetic findings and prognosis in neuroblastoma with emphasis on marker chromosome 1. Cancer. 1989 Jan 1;63(1):126–132. doi: 10.1002/1097-0142(19890101)63:1<126::aid-cncr2820630120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hayes F. A., Green A., Hustu H. O., Kumar M. Surgicopathologic staging of neuroblastoma: prognostic significance of regional lymph node metastases. J Pediatr. 1983 Jan;102(1):59–62. doi: 10.1016/s0022-3476(83)80287-x. [DOI] [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Miyoshi Y., Ichii S., Nagase H., Ando H., Yanagisawa A., Tsuchiya E., Kato Y., Nakamura Y. Frequent somatic mutations of the APC gene in human pancreatic cancer. Cancer Res. 1992 Dec 1;52(23):6696–6698. [PubMed] [Google Scholar]
- Horii A., Nakatsuru S., Miyoshi Y., Ichii S., Nagase H., Kato Y., Yanagisawa A., Nakamura Y. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res. 1992 Jun 1;52(11):3231–3233. [PubMed] [Google Scholar]
- Imamura J., Bartram C. R., Berthold F., Harms D., Nakamura H., Koeffler H. P. Mutation of the p53 gene in neuroblastoma and its relationship with N-myc amplification. Cancer Res. 1993 Sep 1;53(17):4053–4058. [PubMed] [Google Scholar]
- Kaneko Y., Kanda N., Maseki N., Sakurai M., Tsuchida Y., Takeda T., Okabe I., Sakurai M. Different karyotypic patterns in early and advanced stage neuroblastomas. Cancer Res. 1987 Jan 1;47(1):311–318. [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Komuro H., Hayashi Y., Kawamura M., Hayashi K., Kaneko Y., Kamoshita S., Hanada R., Yamamoto K., Hongo T., Yamada M. Mutations of the p53 gene are involved in Ewing's sarcomas but not in neuroblastomas. Cancer Res. 1993 Nov 1;53(21):5284–5288. [PubMed] [Google Scholar]
- Look A. T., Hayes F. A., Nitschke R., McWilliams N. B., Green A. A. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N Engl J Med. 1984 Jul 26;311(4):231–235. doi: 10.1056/NEJM198407263110405. [DOI] [PubMed] [Google Scholar]
- Look A. T., Hayes F. A., Shuster J. J., Douglass E. C., Castleberry R. P., Bowman L. C., Smith E. I., Brodeur G. M. Clinical relevance of tumor cell ploidy and N-myc gene amplification in childhood neuroblastoma: a Pediatric Oncology Group study. J Clin Oncol. 1991 Apr;9(4):581–591. doi: 10.1200/JCO.1991.9.4.581. [DOI] [PubMed] [Google Scholar]
- MacMillan R. W., Blanc W. B., Santulli T. V. Maturation of neuroblastoma to ganglioneuroma in lymph nodes. J Pediatr Surg. 1976 Jun;11(3):461–462. doi: 10.1016/s0022-3468(76)80204-7. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y., Nagase H., Ando H., Horii A., Ichii S., Nakatsuru S., Aoki T., Miki Y., Mori T., Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992 Jul;1(4):229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Nakatsuru S., Yanagisawa A., Ichii S., Tahara E., Kato Y., Nakamura Y., Horii A. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992 Nov;1(8):559–563. doi: 10.1093/hmg/1.8.559. [DOI] [PubMed] [Google Scholar]
- Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991 Aug 9;253(5020):665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- Noble J. R., Willetts K. E., Mercer W. E., Reddel R. R. Effects of exogenous wild-type p53 on a human lung carcinoma cell line with endogenous wild-type p53. Exp Cell Res. 1992 Dec;203(2):297–304. doi: 10.1016/0014-4827(92)90002-p. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Robertson G. P., Coleman A. B., Lugo T. G. Mechanisms of human melanoma cell growth and tumor suppression by chromosome 6. Cancer Res. 1996 Apr 1;56(7):1635–1641. [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Klempnauer K. H., Varmus H. E., Bishop J. M., Gilbert F., Brodeur G., Goldstein M., Trent J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature. 1983 Sep 15;305(5931):245–248. doi: 10.1038/305245a0. [DOI] [PubMed] [Google Scholar]
- Seeger R. C., Brodeur G. M., Sather H., Dalton A., Siegel S. E., Wong K. Y., Hammond D. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985 Oct 31;313(18):1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Masayuki I. [Primary cell culture of esophageal cancer as an indicator of biological malignancy]. Hum Cell. 1994 Dec;7(4):193–198. [PubMed] [Google Scholar]
- Srivatsan E. S., Murali V., Seeger R. C. Loss of heterozygosity for alleles on chromosomes 11q and 14q in neuroblastoma. Prog Clin Biol Res. 1991;366:91–98. [PubMed] [Google Scholar]
- Suzuki T., Yokota J., Mugishima H., Okabe I., Ookuni M., Sugimura T., Terada M. Frequent loss of heterozygosity on chromosome 14q in neuroblastoma. Cancer Res. 1989 Mar 1;49(5):1095–1098. [PubMed] [Google Scholar]
- The I., Murthy A. E., Hannigan G. E., Jacoby L. B., Menon A. G., Gusella J. F., Bernards A. Neurofibromatosis type 1 gene mutations in neuroblastoma. Nat Genet. 1993 Jan;3(1):62–66. doi: 10.1038/ng0193-62. [DOI] [PubMed] [Google Scholar]
- Trent J. M., Stanbridge E. J., McBride H. L., Meese E. U., Casey G., Araujo D. E., Witkowski C. M., Nagle R. B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- Vogan K., Bernstein M., Leclerc J. M., Brisson L., Brossard J., Brodeur G. M., Pelletier J., Gros P. Absence of p53 gene mutations in primary neuroblastomas. Cancer Res. 1993 Nov 1;53(21):5269–5273. [PubMed] [Google Scholar]