Abstract
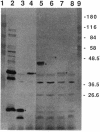
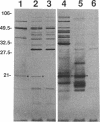
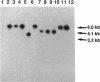
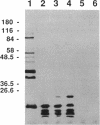
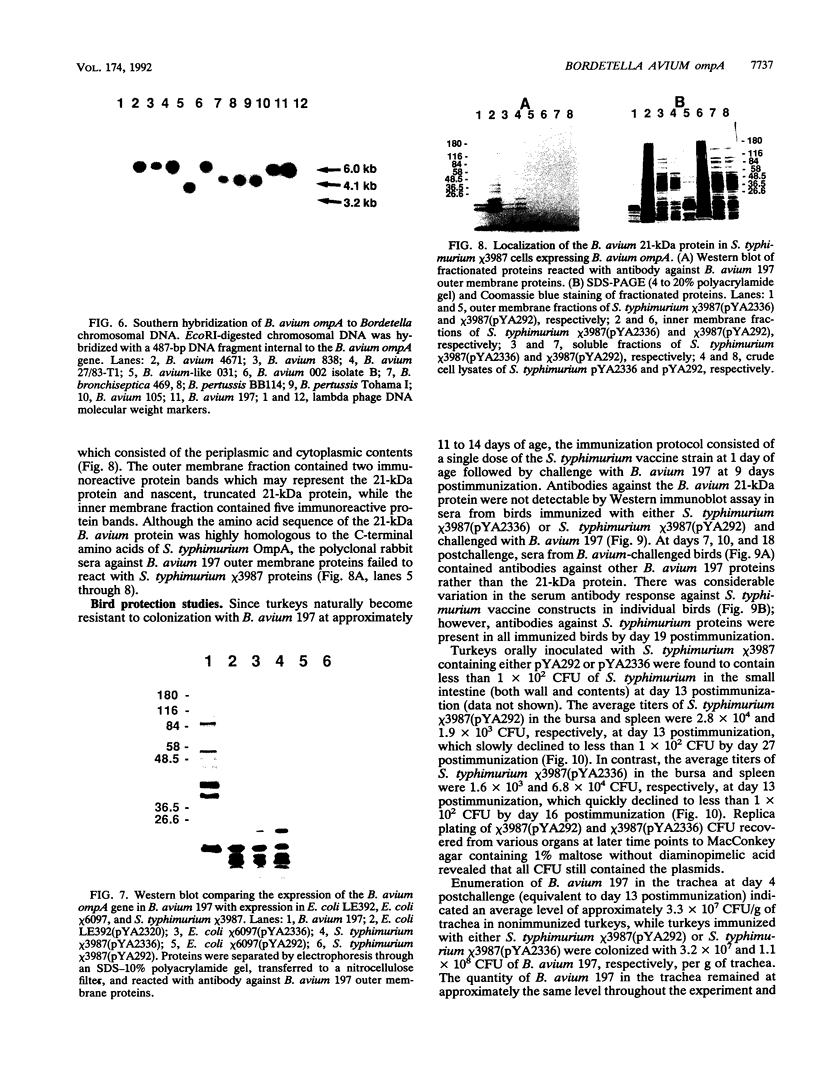
Three gene libraries of Bordetella avium 197 DNA were prepared in Escherichia coli LE392 by using the cosmid vectors pCP13 and pYA2329, a derivative of pCP13 specifying spectinomycin resistance. The cosmid libraries were screened with convalescent-phase anti-B. avium turkey sera and polyclonal rabbit antisera against B. avium 197 outer membrane proteins. One E. coli recombinant clone produced a 56-kDa protein which reacted with convalescent-phase serum from a turkey infected with B. avium 197. In addition, five E. coli recombinant clones were identified which produced B. avium outer membrane proteins with molecular masses of 21, 38, 40, 43, and 48 kDa. At least one of these E. coli clones, which encoded the 21-kDa protein, reacted with both convalescent-phase turkey sera and antibody against B. avium 197 outer membrane proteins. The gene for the 21-kDa outer membrane protein was localized by Tn5seq1 mutagenesis, and the nucleotide sequence was determined by dideoxy sequencing. DNA sequence analysis of the 21-kDa protein revealed an open reading frame of 582 bases that resulted in a predicted protein of 194 amino acids. Comparison of the predicted amino acid sequence of the gene encoding the 21-kDa outer membrane protein with protein sequences in the National Biomedical Research Foundation protein sequence data base indicated significant homology to the OmpA proteins of Shigella dysenteriae, Enterobacter aerogenes, E. coli, and Salmonella typhimurium and to Neisseria gonorrhoeae outer membrane protein III, Haemophilus influenzae protein P6, and Pseudomonas aeruginosa porin protein F. The gene (ompA) encoding the B. avium 21-kDa protein hybridized with 4.1-kb DNA fragments from EcoRI-digested, chromosomal DNA of Bordetella pertussis and Bordetella bronchiseptica and with 6.0- and 3.2-kb DNA fragments from EcoRI-digested, chromosomal DNA of B. avium and B. avium-like DNA, respectively. A 6.75-kb DNA fragment encoding the B. avium 21-kDa protein was subcloned into the Asd+ vector pYA292, and the construct was introduced into the avirulent delta cya delta crp delta asd S. typhimurium chi 3987 for oral immunization of birds. The gene encoding the 21-kDa protein was expressed equivalently in B. avium 197, delta asd E. coli chi 6097, and S. typhimurium chi 3987 and was localized primarily in the cytoplasmic membrane and outer membrane. In preliminary studies on oral inoculation of turkey poults with S. typhimurium chi 3987 expressing the gene encoding the B. avium 21-kDa protein, it was determined that a single dose of the recombinant Salmonella vaccine failed to elicit serum antibodies against the 21-kDa protein and challenge with wild-type B. avium 197 resulted in colonization of the trachea and thymus with B. avium 197.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeila M. A., Saif Y. M. Protection of turkey poults from Bordetella avium infection and disease by pili and bacterins. Avian Dis. 1988 Oct-Dec;32(4):641–649. [PubMed] [Google Scholar]
- Arp L. H., Cheville N. F. Tracheal lesions in young turkeys infected with Bordetella avium. Am J Vet Res. 1984 Oct;45(10):2196–2200. [PubMed] [Google Scholar]
- Arp L. H., Fagerland J. A. Ultrastructural pathology of Bordetella avium infection in turkeys. Vet Pathol. 1987 Sep;24(5):411–418. doi: 10.1177/030098588702400508. [DOI] [PubMed] [Google Scholar]
- Arp L. H., Leyh R. D., Griffith R. W. Adherence of Bordetella avium to tracheal mucosa of turkeys: correlation with hemagglutination. Am J Vet Res. 1988 May;49(5):693–696. [PubMed] [Google Scholar]
- Beck E., Bremer E. Nucleotide sequence of the gene ompA coding the outer membrane protein II of Escherichia coli K-12. Nucleic Acids Res. 1980 Jul 11;8(13):3011–3027. doi: 10.1093/nar/8.13.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhoff H. A., McCorkle F. M., Jr, Brown T. T. Pathogenicity of various isolates of Alcaligenes faecalis for broilers. Avian Dis. 1983 Jul-Sep;27(3):707–713. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Braun G., Cole S. T. DNA sequence analysis of the Serratia marcescens ompA gene: implications for the organisation of an enterobacterial outer membrane protein. Mol Gen Genet. 1984;195(1-2):321–328. doi: 10.1007/BF00332766. [DOI] [PubMed] [Google Scholar]
- Braun G., Cole S. T. Molecular characterization of the gene coding for major outer membrane protein OmpA from Enterobacter aerogenes. Eur J Biochem. 1983 Dec 15;137(3):495–500. doi: 10.1111/j.1432-1033.1983.tb07853.x. [DOI] [PubMed] [Google Scholar]
- Braun G., Cole S. T. The nucleotide sequence coding for major outer membrane protein OmpA of Shigella dysenteriae. Nucleic Acids Res. 1982 Apr 10;10(7):2367–2378. doi: 10.1093/nar/10.7.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E., Cole S. T., Hindennach I., Henning U., Beck E., Kurz C., Schaller H. Export of a protein into the outer membrane of Escherichia coli K12. Stable incorporation of the OmpA protein requires less than 193 amino-terminal amino-acid residues. Eur J Biochem. 1982 Feb;122(1):223–231. doi: 10.1111/j.1432-1033.1982.tb05870.x. [DOI] [PubMed] [Google Scholar]
- Chen R., Henning U. Nucleotide sequence of the gene for the peptidoglycan-associated lipoprotein of Escherichia coli K12. Eur J Biochem. 1987 Feb 16;163(1):73–77. doi: 10.1111/j.1432-1033.1987.tb10738.x. [DOI] [PubMed] [Google Scholar]
- Chen R., Schmidmayr W., Krämer C., Chen-Schmeisser U., Henning U. Primary structure of major outer membrane protein II (ompA protein) of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4592–4596. doi: 10.1073/pnas.77.8.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Zaitlin D., Kado C. I. Design and development of amplifiable broad-host-range cloning vectors: analysis of the vir region of Agrobacterium tumefaciens plasmid pTiC58. Plasmid. 1984 Sep;12(2):111–118. doi: 10.1016/0147-619x(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Bremer E., Hindennach I., Henning U. Characterisation of the promoters for the ompA gene which encodes a major outer membrane protein of Escherichia coli. Mol Gen Genet. 1982;188(3):472–479. doi: 10.1007/BF00330051. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzins A., Chakrabarty A. M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984 Jul;159(1):9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchêne M., Schweizer A., Lottspeich F., Krauss G., Marget M., Vogel K., von Specht B. U., Domdey H. Sequence and transcriptional start site of the Pseudomonas aeruginosa outer membrane porin protein F gene. J Bacteriol. 1988 Jan;170(1):155–162. doi: 10.1128/jb.170.1.155-162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R., Cole S. T. Cloning and molecular characterization of the ompA gene from Salmonella typhimurium. Eur J Biochem. 1983 Aug 15;134(3):497–502. doi: 10.1111/j.1432-1033.1983.tb07594.x. [DOI] [PubMed] [Google Scholar]
- Freudl R., Klose M., Henning U. Export and sorting of the Escherichia coli outer membrane protein OmpA. J Bioenerg Biomembr. 1990 Jun;22(3):441–449. doi: 10.1007/BF00763176. [DOI] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Stierhof Y. D., Gamon K., Hindennach I., Henning U. An outer membrane protein (OmpA) of Escherichia coli K-12 undergoes a conformational change during export. J Biol Chem. 1986 Aug 25;261(24):11355–11361. [PubMed] [Google Scholar]
- Galán J. E., Nakayama K., Curtiss R., 3rd Cloning and characterization of the asd gene of Salmonella typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene. 1990 Sep 28;94(1):29–35. doi: 10.1016/0378-1119(90)90464-3. [DOI] [PubMed] [Google Scholar]
- Gentry-Weeks C. R., Cookson B. T., Goldman W. E., Rimler R. B., Porter S. B., Curtiss R., 3rd Dermonecrotic toxin and tracheal cytotoxin, putative virulence factors of Bordetella avium. Infect Immun. 1988 Jul;56(7):1698–1707. doi: 10.1128/iai.56.7.1698-1707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry-Weeks C. R., Provence D. L., Keith J. M., Curtiss R., 3rd Isolation and characterization of Bordetella avium phase variants. Infect Immun. 1991 Nov;59(11):4026–4033. doi: 10.1128/iai.59.11.4026-4033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibert I., Barbé J. Cyclic AMP stimulates transcription of the structural gene of the outer-membrane protein OmpA of Escherichia coli. FEMS Microbiol Lett. 1990 Mar 15;56(3):307–311. doi: 10.1111/j.1574-6968.1988.tb03197.x. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M., Blake M. S. The DNA sequence of the structural gene of gonococcal protein III and the flanking region containing a repetitive sequence. Homology of protein III with enterobacterial OmpA proteins. J Exp Med. 1987 Feb 1;165(2):471–482. doi: 10.1084/jem.165.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. G., Roberts J. F., Dillman R. C., Simmons D. G. Pathogenesis of change in the upper respiratory tracts of turkeys experimentally infected with an Alcaligenes faecalis isolate. Infect Immun. 1983 Oct;42(1):350–355. doi: 10.1128/iai.42.1.350-355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B. A., Metcalf B. J., Quinn-Dey T., Kirkley D. H., Quataert S. A., Deich R. A. A recombinant non-fatty acylated form of the Hi-PAL (P6) protein of Haemophilus influenzae elicits biologically active antibody against both nontypeable and type b H. influenzae. Infect Immun. 1990 Oct;58(10):3272–3278. doi: 10.1128/iai.58.10.3272-3278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna Z., Fregeau C., Préfontaine G., Brousseau R. Construction of a family of universal expression plasmid vectors. Gene. 1984 Oct;30(1-3):247–250. doi: 10.1016/0378-1119(84)90128-8. [DOI] [PubMed] [Google Scholar]
- Hassan J. O., Curtiss R., 3rd Control of colonization by virulent Salmonella typhimurium by oral immunization of chickens with avirulent delta cya delta crp S. typhimurium. Res Microbiol. 1990 Sep-Oct;141(7-8):839–850. doi: 10.1016/0923-2508(90)90119-b. [DOI] [PubMed] [Google Scholar]
- Hellwig D. H., Arp L. H., Fagerland J. A. A comparison of outer membrane proteins and surface characteristics of adhesive and non-adhesive phenotypes of Bordetella avium. Avian Dis. 1988 Oct-Dec;32(4):787–792. [PubMed] [Google Scholar]
- Hellwig D. H., Arp L. H. Identification of Bordetella avium antigens recognized after experimental inoculation in turkeys. Am J Vet Res. 1990 Aug;51(8):1188–1191. [PubMed] [Google Scholar]
- Hinz K. H., Glünder G., Lüders H. Acute respiratory disease in turkey poults caused by Bordetella bronchiseptica-like bacteria. Vet Rec. 1978 Sep 16;103(12):262–263. doi: 10.1136/vr.103.12.262. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M. W., Saif Y. M. Pili of Bordetella avium: expression, characterization, and role in in vitro adherence. Avian Dis. 1987 Apr-Jun;31(2):277–286. [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Marshall D. R., Simmons D. G., Gray J. G. An Alcaligenes faecalis isolate from turkeys: pathogenicity in selected avian and mammalian species. Am J Vet Res. 1985 May;46(5):1181–1184. [PubMed] [Google Scholar]
- Matthews-Greer J. M., Gilleland H. E., Jr Outer membrane protein F (porin) preparation of Pseudomonas aeruginosa as a protective vaccine against heterologous immunotype strains in a burned mouse model. J Infect Dis. 1987 Jun;155(6):1282–1291. doi: 10.1093/infdis/155.6.1282. [DOI] [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Gene structure of the OmpA protein, a major surface protein of Escherichia coli required for cell-cell interaction. J Mol Biol. 1980 Nov 5;143(3):317–328. doi: 10.1016/0022-2836(80)90193-x. [DOI] [PubMed] [Google Scholar]
- Movva R. N., Green P., Nakamura K., Inouye M. Interaction of cAMP receptor protein with the ompA gene, a gene for a major outer membrane protein of Escherichia coli. FEBS Lett. 1981 Jun 15;128(2):186–190. doi: 10.1016/0014-5793(81)80077-4. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Granoff D. M. Purification and partial characterization of outer membrane proteins P5 and P6 from Haemophilus influenzae type b. Infect Immun. 1985 Sep;49(3):544–549. doi: 10.1128/iai.49.3.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag D. K., Huang H. V., Berg D. E. Bidirectional chain-termination nucleotide sequencing: transposon Tn5seq1 as a mobile source of primer sites. Gene. 1988 Apr 15;64(1):135–145. doi: 10.1016/0378-1119(88)90487-8. [DOI] [PubMed] [Google Scholar]
- Nelson M. B., Apicella M. A., Murphy T. F., Vankeulen H., Spotila L. D., Rekosh D. Cloning and sequencing of Haemophilus influenzae outer membrane protein P6. Infect Immun. 1988 Jan;56(1):128–134. doi: 10.1128/iai.56.1.128-134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. Protein secretion in Escherichia coli. Annu Rev Microbiol. 1985;39:615–648. doi: 10.1146/annurev.mi.39.100185.003151. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Saif Y. M., Moorhead P. D., Dearth R. N., Jackwood D. J. Observations on Alcaligenes faecalis infection in turkeys. Avian Dis. 1980 Jul-Sep;24(3):665–684. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Kapelner S., Sommer S. S. Formamide can dramatically improve the specificity of PCR. Nucleic Acids Res. 1990 Dec 25;18(24):7465–7465. doi: 10.1093/nar/18.24.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Simmons D. G., Davis D. E., Rose L. P., Gray J. G., Luginbuhl G. H. Alcaligenes faecalis-associated respiratory disease of chickens. Avian Dis. 1981 Jul-Sep;25(3):610–613. [PubMed] [Google Scholar]
- Simmons D. G., Gray J. G., Rose L. P., Dillman R. C., Miller S. E. Isolation of an etiologic agent of acute respiratory disease (rhinotracheitis) of turkey poults. Avian Dis. 1979 Jan-Mar;23(1):194–203. [PubMed] [Google Scholar]
- Stroynowski I., Kuroda M., Yanofsky C. Transcription termination in vitro at the tryptophan operon attenuator is controlled by secondary structures in the leader transcript. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2206–2210. doi: 10.1073/pnas.80.8.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji Y., Gennity J., Pollitt S., Inouye M. Effect of OmpA signal peptide mutations on OmpA secretion, synthesis, and assembly. J Bacteriol. 1991 Mar;173(6):1997–2005. doi: 10.1128/jb.173.6.1997-2005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Tiemeier D. C., Polsky F., Edgell M. H., Seidman J. G., Leder A., Enquist L. W., Norman B., Leder P. Cloning specific segments of the mammalian genome: bacteriophage lambda containing mouse globin and surrounding gene sequences. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4406–4410. doi: 10.1073/pnas.74.10.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinge S. A., Curtiss R., 3rd Conservation of Salmonella typhimurium virulence plasmid maintenance regions among Salmonella serovars as a basis for plasmid curing. Infect Immun. 1990 Sep;58(9):3084–3092. doi: 10.1128/iai.58.9.3084-3092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Alstine W. G., Arp L. H. Histologic evaluation of lung and bronchus-associated lymphoid tissue in young turkeys infected with Bordetella avium. Am J Vet Res. 1988 Jun;49(6):835–839. [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]