Abstract
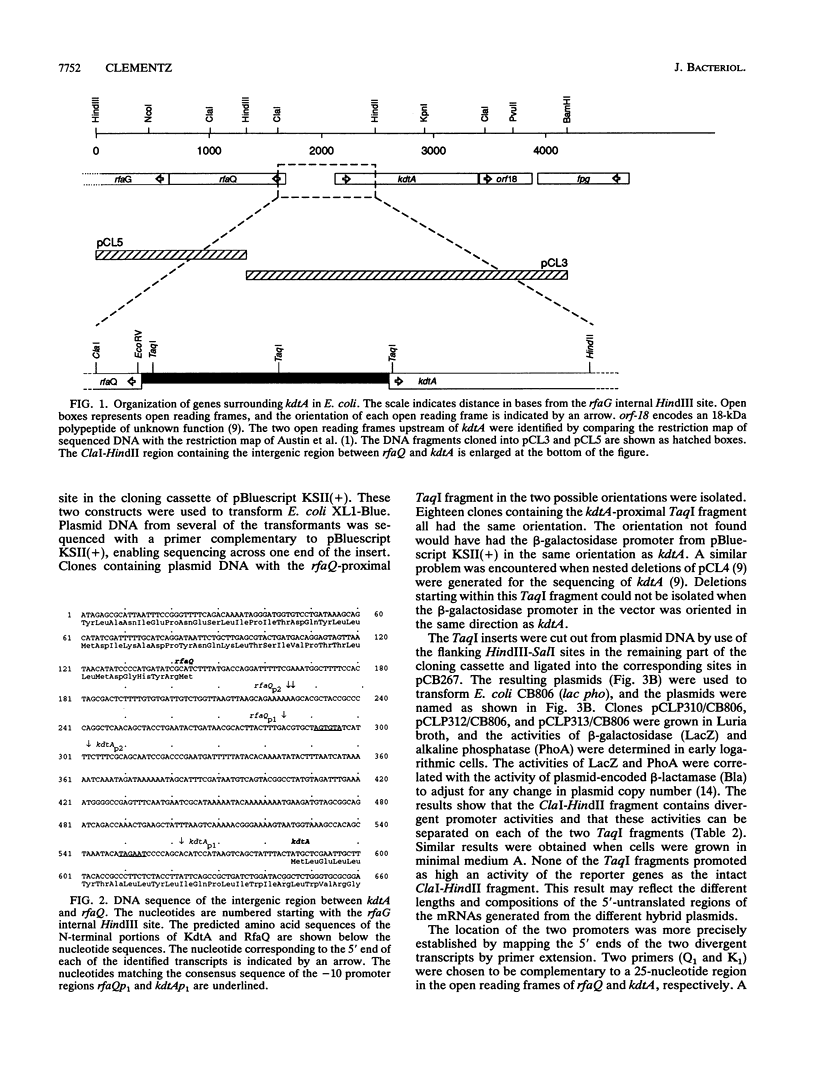
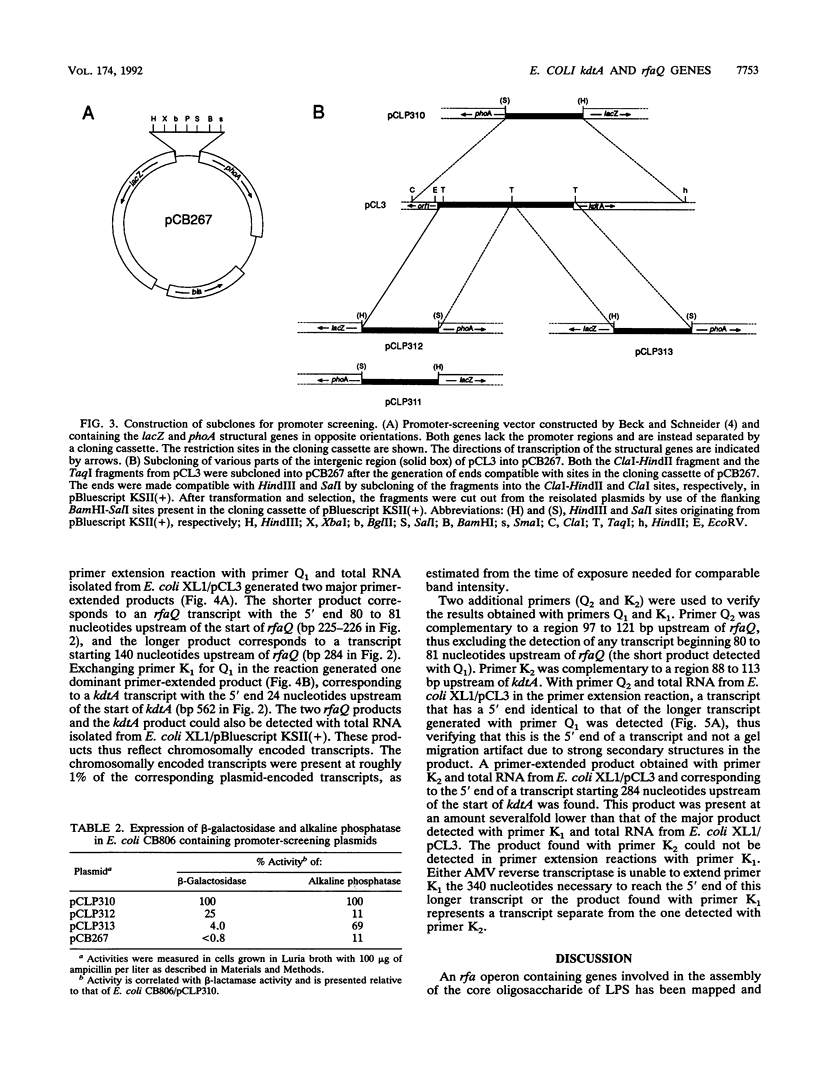
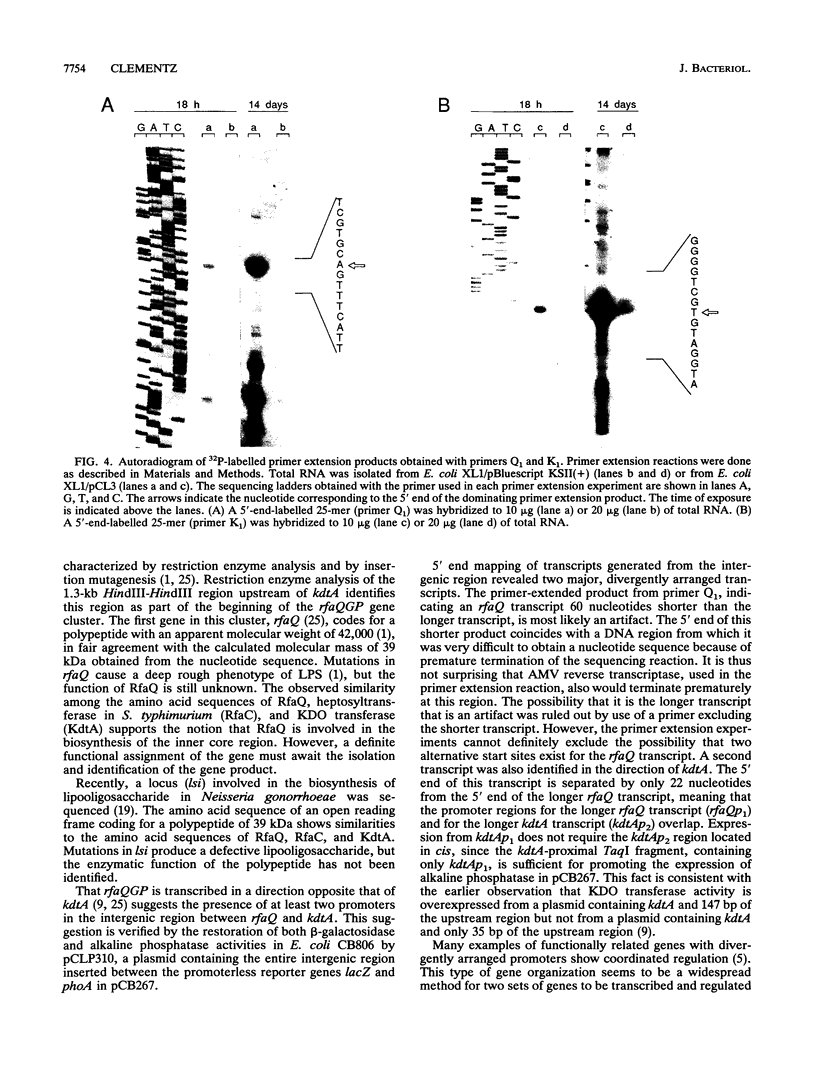
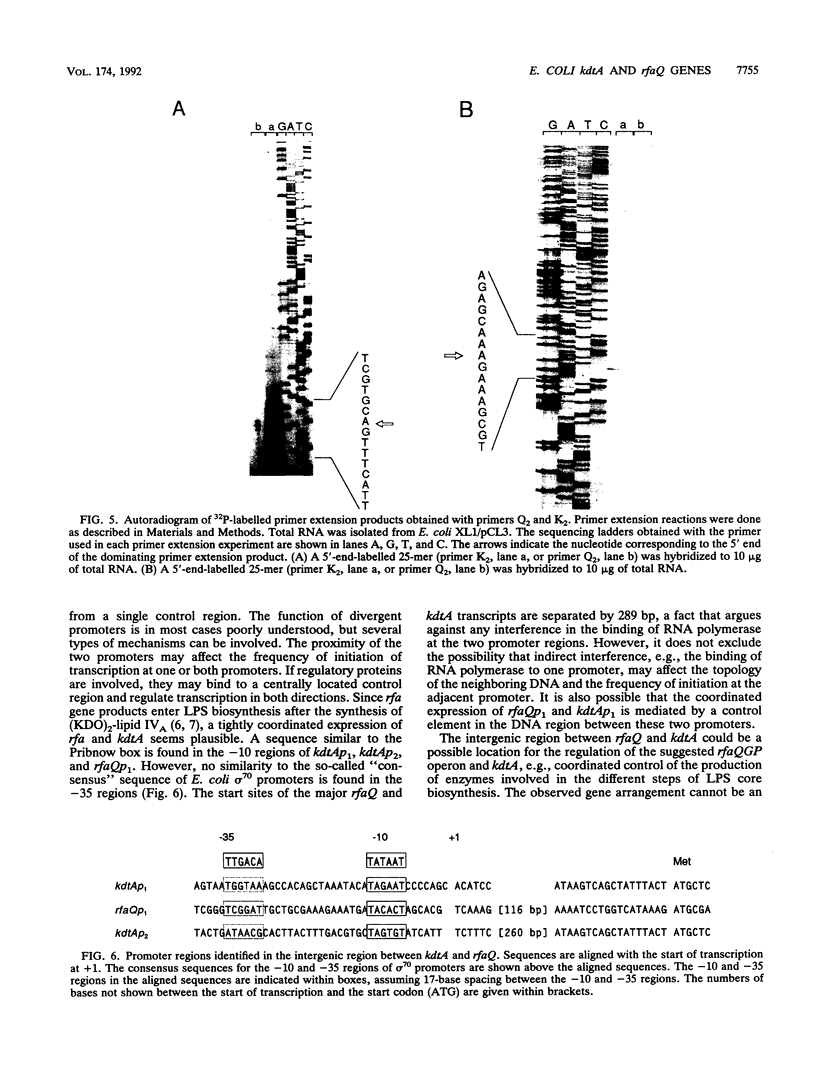
The gene kdtA in Escherichia coli codes for 3-deoxy-D-manno-octulosonic acid transferase, the enzyme responsible for attachment of the two 3-deoxy-D-manno-octulosonic acid residues that constitute the link between lipid A and the core oligosaccharide of the lipopolysaccharide. Cloning and subsequent sequencing of the region upstream of kdtA revealed an open reading frame identified as the first gene (rfaQ) in an rfa gene cluster. The kdtA and rfaQ transcripts were identified, and the 5' ends of the transcripts were mapped by primer extension. Two main, divergently arranged promoters were found. These promoters generated transcripts with 5' ends separated by 289 bases. That the two divergent transcripts from the identified promoters represent the kdtA and rfaQ transcripts was confirmed by fusing different parts of the intergenic region between the promoterless lacZ and phoA genes in promoter-screening plasmid pCB267.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin E. A., Graves J. F., Hite L. A., Parker C. T., Schnaitman C. A. Genetic analysis of lipopolysaccharide core biosynthesis by Escherichia coli K-12: insertion mutagenesis of the rfa locus. J Bacteriol. 1990 Sep;172(9):5312–5325. doi: 10.1128/jb.172.9.5312-5325.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer D. E., Dynan W. S. Simian virus 40 major late promoter: a novel tripartite structure that includes intragenic sequences. Mol Cell Biol. 1988 May;8(5):2021–2033. doi: 10.1128/mcb.8.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. F., Warren R. A. Divergent promoters, a common form of gene organization. Microbiol Rev. 1988 Sep;52(3):318–326. doi: 10.1128/mr.52.3.318-326.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozek K. A., Hosaka K., Robertson A. D., Raetz C. R. Biosynthesis of lipopolysaccharide in Escherichia coli. Cytoplasmic enzymes that attach 3-deoxy-D-manno-octulosonic acid to lipid A. J Biol Chem. 1989 Apr 25;264(12):6956–6966. [PubMed] [Google Scholar]
- Brozek K. A., Raetz C. R. Biosynthesis of lipid A in Escherichia coli. Acyl carrier protein-dependent incorporation of laurate and myristate. J Biol Chem. 1990 Sep 15;265(26):15410–15417. [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clementz T., Raetz C. R. A gene coding for 3-deoxy-D-manno-octulosonic-acid transferase in Escherichia coli. Identification, mapping, cloning, and sequencing. J Biol Chem. 1991 May 25;266(15):9687–9696. [PubMed] [Google Scholar]
- Coleman J., Raetz C. R. First committed step of lipid A biosynthesis in Escherichia coli: sequence of the lpxA gene. J Bacteriol. 1988 Mar;170(3):1268–1274. doi: 10.1128/jb.170.3.1268-1274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeger E. S., Rothfield L. I. Cloning of genes for bacterial glycosyltransferases. I. Selection of hybrid plasmids carrying genes for two glucosyltransferases. J Biol Chem. 1979 Feb 10;254(3):804–810. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Klotsky R. A., Schwartz I. Measurement of cat expression from growth-rate-regulated promoters employing beta-lactamase activity as an indicator of plasmid copy number. Gene. 1987;55(1):141–146. doi: 10.1016/0378-1119(87)90257-5. [DOI] [PubMed] [Google Scholar]
- MacLachlan P. R., Kadam S. K., Sanderson K. E. Cloning, characterization, and DNA sequence of the rfaLK region for lipopolysaccharide synthesis in Salmonella typhimurium LT2. J Bacteriol. 1991 Nov;173(22):7151–7163. doi: 10.1128/jb.173.22.7151-7163.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D., Bathgate A. J., Poxton I. R. Monoclonal antibodies as probes for detecting lipopolysaccharide expression on Escherichia coli from different growth conditions. J Gen Microbiol. 1991 Dec;137(12):2741–2751. doi: 10.1099/00221287-137-12-2741. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. T., Pradel E., Schnaitman C. A. Identification and sequences of the lipopolysaccharide core biosynthetic genes rfaQ, rfaP, and rfaG of Escherichia coli K-12. J Bacteriol. 1992 Feb;174(3):930–934. doi: 10.1128/jb.174.3.930-934.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petricoin E. F., 3rd, Danaher R. J., Stein D. C. Analysis of the lsi region involved in lipooligosaccharide biosynthesis in Neisseria gonorrhoeae. J Bacteriol. 1991 Dec;173(24):7896–7902. doi: 10.1128/jb.173.24.7896-7902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel E., Schnaitman C. A. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J Bacteriol. 1991 Oct;173(20):6428–6431. doi: 10.1128/jb.173.20.6428-6431.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Resnekov O., Rutberg L., von Gabain A. Changes in the stability of specific mRNA species in response to growth stage in Bacillus subtilis. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8355–8359. doi: 10.1073/pnas.87.21.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., Parker C. T., Klena J. D., Pradel E. L., Pearson N. B., Sanderson K. E., MacClachlan P. R. Physical maps of the rfa loci of Escherichia coli K-12 and Salmonella typhimurium. J Bacteriol. 1991 Dec;173(23):7410–7411. doi: 10.1128/jb.173.23.7410-7411.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Beck C. F. New expression vectors for identifying and testing signal structures for initiation and termination of transcription. Methods Enzymol. 1987;153:452–461. doi: 10.1016/0076-6879(87)53071-3. [DOI] [PubMed] [Google Scholar]




