Abstract
Phytochelatins (PCs) with good binding affinities for a wide range of heavy metals were exploited to develop microbial sorbents for cadmium removal. PC synthase from Schizosaccharomyces pombe (SpPCS) was overexpressed in Escherichia coli, resulting in PC synthesis and 7.5-times-higher Cd accumulation. The coexpression of a variant γ-glutamylcysteine synthetase desensitized to feedback inhibition (GshI*) increased the supply of the PC precursor glutathione, resulting in further increases of 10- and 2-fold in PC production and Cd accumulation, respectively. A Cd transporter, MntA, was expressed with SpPCS and GshI* to improve Cd uptake, resulting in a further 1.5-fold increase in Cd accumulation. The level of Cd accumulation in this recombinant E. coli strain (31.6 μmol/g [dry weight] of cells) was more than 25-fold higher than that in the control strain.
Widespread pollution by heavy metals generated from various industrial and agricultural activities has serious adverse effects on human health and ecosystems (12). The increased public concern over these heavy metals has caused more-stringent control of the allowable limits in drinking water and soil. Although conventional technologies are adequate to remove the bulk of heavy-metal contamination, they are often inadequate to reduce heavy-metal concentrations to acceptable regulatory standards (8). Bioremediation based on genetically engineered bacteria is an emerging technology that is receiving more attention as an inexpensive and efficient way of cleaning up toxic-metal contamination (10). In particular, the production of metal-binding peptides such as metallothioneins and phytochelatins (PCs) has been shown to confer enhanced heavy-metal-binding capabilities (1, 2, 18).
PCs are naturally occurring peptides consisting of the repeating γ-Glu-Cys dipeptide unit terminated by a Gly residue (6, 21). The presence of a γ bond between glutamic acid and cysteine indicates that the synthesis of PCs cannot occur via the ribosomes. PC biosynthesis, indeed, proceeds through the transfer of γ-Glu-Cys from glutathione (GSH) to another GSH or other PCs (21) by the enzyme PC synthase (PCS) when this enzyme is activated by heavy metals such as Cd, Cu, Hg, and Pb. PCs are known to bind heavy metals such as Cd, Hg, As, and Pb, especially cadmium, with high affinity through thiolate complexes (7, 16). Recently, the genes coding for PCS have been cloned from plants and fungi and functionally expressed in Escherichia coli (15). The intracellular cadmium content of the E. coli strain expressing PCS increased more substantially than that of the control strain. A direct correlation between PC content and metal accumulation was observed, indicating that PC is primarily responsible for the metal sequestration.
Although the initial results of the previous study (15) demonstrated the possibility of heavy-metal remediation using PC-producing cells, a severe drop in the GSH content suggests that the GSH supply may be the limiting step in PC synthesis. In E. coli, the biosynthesis of GSH proceeds via γ-glutamylcysteine synthetase (GshI) with the conversion of glutamate and cysteine into γ-glutamylcysteine (γ-EC), which is then converted into GSH by GSH synthetase (9). Since the synthesis of γ-EC is reported to be rate limiting (5), one potential strategy to increase the supply of GSH is to overexpress GshI.
In this work, a variant of GshI, GshI*, which is desensitized to feedback inhibition by GSH (11), was overexpressed in E. coli, resulting in a significant increase in PC production and a corresponding increase in heavy-metal accumulation. Further improvement in Cd accumulation was achieved by the coexpression of a Cd transporter, MntA, suggesting that PC-mediated Cd accumulation can be optimized by manipulating the GSH and PC synthesis pathways in a rational manner.
Expression of different PCSs and effects on cadmium accumulation.
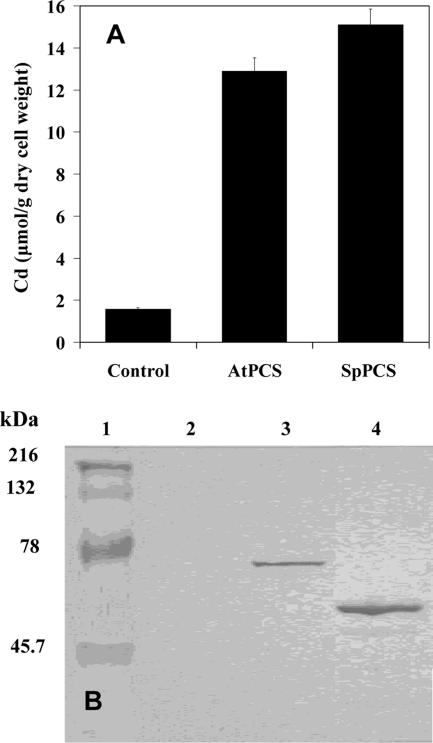
Previously, the gene for Arabidopsis thaliana PCS (AtPCS) was functionally expressed in E. coli, resulting in a significant increase in the cellular cadmium content (15). To investigate whether other PCSs could function similarly, the effect of PCS from Schizosaccharomyces pombe (SpPCS) (3) on cadmium accumulation was compared with that of AtPCS. The expression of both PCS genes was under the control of a T5 tac promoter, and a sequence encoding a hexahistidine tag was added to the end of each gene corresponding to the C terminus of the product to allow easy detection with an anti-His tag antibody (see the supplemental material for details). Transformed JM109 cells were grown in Luria-Bertani medium with appropriate antibiotics to an optical density of 0.5 before the addition of 20 μM CdCl2 and 0.8 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG). For the evaluation of whole-cell metal contents, cells were washed three times with 5 mM HEPES buffer containing 0.8% NaCl before drying at 65°C for 24 h. The dried cell pellets were digested with 100 μl of concentrated nitric acid for 2 days according to a method modified from that of Sriprang et al. (19). The total cadmium contents were measured using atomic absorption spectrometry with an AAnalyst 800 spectrometer (PerkinElmer Inc., Waltham, MA). Although the expression of either PCS resulted in an increase of more than eightfold in the intracellular cadmium content over that of the control strain (Fig. 1A), the level of accumulation in cells expressing SpPCS was 20% higher than that in cells expressing AtPCS. This difference in cadmium accumulation can be attributed partially to the twofold-lower level of expression of AtPCS than of SpPCS (Fig. 1B) as determined by Western blotting (14) and densitometry. Cells expressing SpPCS exhibited a level of Cd accumulation (14.9 μmol/g [dry weight] of cells) twofold higher than the levels achieved in a previous study using AtPCS (7.2 μmol/g [dry weight] of cells) (15). Owing to the higher level of Cd2+ accumulation and the improved PCS production, cells expressing SpPCS were investigated further.
FIG. 1.
Comparison of E. coli JM109 cells expressing AtPCS or SpPCS. (A) Cadmium accumulation in E. coli cells harboring either pQE60 (control), pQE-AtPCS (AtPCS), or pQE-SpPCS (SpPCS). The Cd content was measured by atomic absorption spectrometry. Data shown are the mean values (+ standard deviations) obtained from three independent experiments. (B) Western blot analysis of PCS expression in E. coli cells harboring either pQE60 (lane 2), pQE-AtPCS (lane 3), or pQE-SpPCS (lane 4). The molecular weight marker is shown in lane 1. The production of PCS was detected by an anti-His tag antibody, and the intensity was quantified using densitometry.
Effect of GshI* overexpression on the intracellular cadmium, PC, GSH, and γ-EC contents.
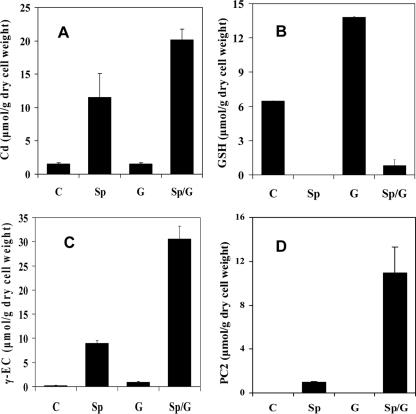
To investigate the effect of SpPCS expression on the intracellular thiol contents, the levels of γ-EC, GSH, and PCs were measured. The derivatization procedure with monobromobimane using fluorescence detection was adopted from Sneller et al. (17). The expression of SpPCS resulted in the synthesis of different forms of PC (such as PC2, PC3, and PC4), with PC2 being the dominant species (data not shown). Unlike control cells without SpPCS expression, cells expressing SpPCS no longer contained detectable intracellular GSH (Fig. 2B), indicating that all the available GSH had been used for PC synthesis. These results strongly suggest that the supply of GSH is limiting for PC synthesis. In contrast, the level of γ-EC actually increased in cells expressing SpPCS (Fig. 2C). Since the enzyme responsible for γ-EC synthesis, GshI, is reported to be subject to feedback inhibition by GSH (4), this increased level of γ-EC may be the result of elevated GshI activity in the absence of GSH.
FIG. 2.
Intracellular Cd (A), GSH (B), γ-EC (C), and PC2 (D) contents of E. coli strain JM109 harboring either pQE60 (C), pQE-SpPCS (Sp), pMMB-gshI (G), or both pQE-SpPCS and pMMB-gshI (Sp/G). Data shown are the mean values (+ standard deviations) obtained from three independent experiments.
To test whether increasing the GSH supply could improve PC synthesis, a variant form of the rate-limiting enzyme involved in the biosynthesis of GSH, GshI*, which was desensitized to feedback inhibition, was expressed with SpPCS. Plasmid pMMB-gshI (13), which contained the GshI* gene under the control of a tac promoter, was used for GshI* overexpression alone. The intracellular GSH and γ-EC contents of cells with and without GshI* overexpression were compared. The introduction of pMMB-gshI into E. coli strain JM109 resulted in two- and sixfold increases in the intracellular GSH and γ-EC contents, respectively (Fig. 2B and C). The expression of GshI* with SpPCS resulted in a 10-fold increase in the PC2 content (Fig. 2D). This result confirms that increasing the supply of GSH can effectively alleviate the bottleneck in PC synthesis. Interestingly, the intracellular γ-EC content also increased by 200-fold compared to that of the control, while only a small amount of GSH was detected (Fig. 2B and C). This result suggests that at the elevated level of GshI* and PCS expression, the conversion of γ-EC into GSH by GSH synthetase becomes rate limiting. Unlike the intracellular PC2 content that increased by 10-fold, the level of cadmium accumulation increased by only a further twofold over that of cells expressing only SpPCS, suggesting that other factors such as cadmium uptake may be limiting (Fig. 2A). It has been reported previously that a recombinant E. coli strain harboring the manganese transport gene (mntA) could significantly increase specific cadmium accumulation due to the enhanced uptake (6); a similar strategy was used to further improve the bioaccumulation of cadmium.
Coexpression of MntA, GshI*, and SpPCS further enhances the intracellular cadmium content.
To investigate whether the overall Cd accumulation could be further enhanced by increasing Cd transport, the Cd transporter MntA was overexpressed along with GshI* and SpPCS. Two separate vectors, one (pQE-SpPCS-GshI*) harboring the genes coding for GshI* and SpPCS as an operon and a second carrying the mntA gene on pZH3-5 (6), were used. Interestingly, the PC2 levels in cells carrying the synthetic operon were fourfold lower than those in the previously tested cells in which GshI* and SpPCS were expressed separately (see Fig. S1 in the supplemental material) due to the lower level of SpPCS expression. However, the intracellular Cd contents of cells harboring the synthetic operon remained the same, and the co-overexpression of MntA further increased the intracellular Cd accumulation by 1.5-fold. The resulting level of Cd accumulation (31.6 μmol/g [dry weight] of cells) was more than 25-fold higher than that in the control strain. This result indicates that the uptake of Cd is indeed a key rate-limiting step in the overall Cd accumulation.
It should be noted that Wawrzynska et al. (20) recently reported the coexpression of GshI, SpPCS, and serine acetyltransferase in E. coli. However, Cd accumulation was increased by only fivefold, and no quantitative analysis of the nonprotein thiols was reported. This lower level of Cd enhancement was likely a result of using the wild-type GshI, which is subjected to feedback inhibition by GSH. In the present study, we thoroughly analyzed different bottlenecks in PC synthesis and Cd uptake to achieve high levels of whole-cell Cd accumulation. These levels of accumulation could potentially be further improved by additional manipulations of Cd uptake and PC synthesis.
In conclusion, the overexpression of PCS and GshI* in E. coli improves not only the intracellular PC content but also the subsequent Cd accumulation. Furthermore, the coexpression of MntA, SpPCS, and GshI* leads to an additional increase in Cd accumulation. Because of the high level of heavy-metal accumulation afforded by this strategy, the engineered strains can be easily adapted in immobilized bioreactors for wastewater cleanup. This potential is particularly attractive if resting cells that produce a high level of PCs and are optimized for Cd uptake can be employed. It has been demonstrated previously that resting cells expressing MntA and metallothionein can be used for selective Cd removal (6). The ability of resting cells expressing MntA and SpPCS for effective Cd removal will be similarly investigated.
Supplementary Material
Acknowledgments
This work was supported by grants from the NSF (BES0422791 and BES0329482).
We thank Thomas K. Wood, Texas A&M University, and David Wilson, Cornell University, for plasmids pMMB-gshI and pZH3-5, respectively.
Footnotes
Published ahead of print on 3 August 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bae, W., R. Mehra, A. Mulchandani, and W. Chen. 2001. Genetic engineering of Escherichia coli for enhanced bioaccumulation of mercury. Appl. Environ. Microbiol. 67:5335-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae, W., W. Chen, A. Mulchandani, and R. Mehra. 2000. Enhanced bioaccumulation of heavy metal by bacterial cells displaying synthetic phytochelatins. Biotechnol. Bioeng. 70:518-524. [DOI] [PubMed] [Google Scholar]
- 3.Clemens, S., E. J. Kim, D. Neumann, and J. I. Schroeder. 1999. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 18:3325-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao, Z., S. Chen, and D. B. Wilson. 1999. Cloning, expression, and characterization of cadmium and manganese uptake genes from Lactobacillus plantarum. Appl. Environ. Microbiol. 65:4746-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly, B. S., W. E. Antholine, and W. Griffith. 2002. Escherichia coli γ-glutamylcysteine synthetase. Two active site metal ions affect substrate and inhibitor binding. J. Biol. Chem. 277:50-58. [DOI] [PubMed] [Google Scholar]
- 6.Kim, S. K., B. S. Lee, D. B. Wilson, and E. K. Kim. 2005. Selective cadmium accumulation using recombinant Escherichia coli. J. Biosci. Bioeng. 99:109-114. [DOI] [PubMed] [Google Scholar]
- 7.Maitani, T., H. Kubota, K. Sato, and T. Yamada. 1996. The composition of metals bound to class III metallothionein (phytochelatin and its desglycyl peptide) induced by various metals in root cultures of Rubia tinctorum. Plant Physiol. 110:1145-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik, A. 2004. Metal bioremediation through growing cells. Environ. Int. 30:261-278. [DOI] [PubMed] [Google Scholar]
- 9.Meister, A. 1995. Glutathione biosynthesis and its inhibition. Methods Enzymol. 252:26-30. [DOI] [PubMed] [Google Scholar]
- 10.Mejare, M., and L. Bulow. 2001. Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol. 19:67-73. [DOI] [PubMed] [Google Scholar]
- 11.Murata, K., and A. Kimura. 1982. Cloning of a gene responsible for the biosynthesis of glutathione in Escherichia coli B. Appl. Environ. Microbiol. 44:1444-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nriagu, J. O., and J. M. Pacyna. 1988. Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134-139. [DOI] [PubMed] [Google Scholar]
- 13.Rui, L., Y. M. Kwon, K. F. Reardon, and T. K. Wood. 2004. Metabolic pathway engineering to enhance aerobic degradation of chlorinated ethenes and to reduce their toxicity by cloning a novel glutathione S-transferase, an evolved toluene ο-monooxygenase, and γ-glutamylcysteine synthetase. Environ. Microbiol. 6:491-500. [DOI] [PubMed] [Google Scholar]
- 14.Sambrook, J., E. F. Fritsch, and T. Maniatis. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 15.Sauge-Merle, S., S. Cuine, P. Carrier, C. Lecomte-Pradines, D. T. Luu, and G. Peltier. 2003. Enhanced toxic metal accumulation in engineered bacterial cells expressing Arabidopsis thaliana phytochelatin synthase. Appl. Environ. Microbiol. 69:490-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmoger, M. E. V., M. Oven, and E. Grill. 2000. Detoxification of arsenic by phytochelatins in plants. Plant Physiol. 122:793-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sneller, F. E. C., L. M. Heerwaarden, P. L. M. Koevoets, R. Vooijs, H. Schat, and J. A. C. Verkleij. 2000. Derivatization of phytochelatins from Silence vulgaris, induced upon exposure to arsenate and cadmium: comparison of derivatization with Ellman's reagent and monobromobimane. J. Agric. Food. Chem. 48:4014-4019. [DOI] [PubMed] [Google Scholar]
- 18.Sousa, C., P. Kotrba, T. Ruml, A. Cebolla, and V. D. Lorenzo. 1998. Metalloadsorption by Escherichia coli cells displaying yeast and mammalian metallothioneins anchored to the outer membrane protein LamB. J. Bacteriol. 180:2280-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sriprang, R., M. Hayashi, H. Ono, M. Takagi, K. Hirata, and Y. Murooka. 2003. Enhanced accumulation of Cd2+ by a Mesorhizobium sp. transformed with a gene from Arabidopsis thaliana coding for phytochelatin synthase. Appl. Environ. Microbiol. 69:1791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wawrzynska, A., D. Gaganidze, E. Kopera, K. Piatek, W. Bal, and A. Sirko. 2005. Overexpression of genes involved in phytochelatin biosynthesis in Escherichia coli: effects on growth, cadmium accumulation and thiol level. Acta Biochim. Pol. 52:109-116. [PubMed] [Google Scholar]
- 21.Zenk, M. H. 1996. Heavy metal detoxification in higher plants: a review. Gene 179:21-30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.