Abstract
In the adult hippocampus, two different forms of GABAA receptor-mediated inhibition have been identified: phasic and tonic. The first is due to the activation of GABAA receptors facing the presynaptic releasing sites, whereas the second is due to the activation of receptors localized away from the synapses. Because of their high affinity and low desensitization rate, extrasynaptic receptors are persistently able to sense low concentrations of GABA. Here we show that, early in postnatal life, between postnatal day (P) 2 and P6, CA1 and CA3 pyramidal cells but not stratum radiatum interneurons, express a tonic GABAA-mediated conductance. Block of the neuronal GABA transporter GAT-1 slightly enhanced the persistent GABA conductance in principal cells but not in GABAergic interneurons. However, in adulthood, a tonic GABAA-mediated conductance could be revealed in stratum radiatum interneurons, indicating that the ability of these cells to sense ambient GABA levels is developmentally regulated. Pharmacological analysis of the tonic conductance in principal cells demonstrated the involvement of β2/β3, α5 and γ2 GABAA receptor subunits. Removal of the tonic depolarizing action of GABA with picrotoxin, reduced the excitability and the glutamatergic drive of principal cells but did not modify the excitability of stratum radiatum interneurons. The increased cell excitability and synaptic activity following the activation of extrasynaptic GABAA receptors by ambient GABA would facilitate the induction of giant depolarizing potentials.
In the adult CNS, the efficiency of GABAergic signalling relies on the temporally and spatially regulated expression of GABAA receptors which mediate two distinct forms of inhibition: phasic and tonic. The first form consists of fast inhibitory postsynaptic potentials or currents (IPSPs or IPSCs) regulating point-to-point communication between neurons. The second consists of a persistent inhibitory conductance that plays a crucial role in regulating the membrane potential and cell excitability (Semyanov et al. 2004; Cavelier et al. 2005; Farrant & Nusser, 2005). In the case of phasic inhibition, synaptic receptors, facing the presynaptic release sites, are activated by a brief exposure to a high concentration of GABA released by exocytosis of presynaptic vesicles. Once released, GABA diffuses throughout the neuropil before being taken up by selective plasma membrane transporters which contribute to the later clearance of the neurotransmitter (Cherubini & Conti, 2001; Conti et al. 2004).
In the case of tonic inhibition, extrasynaptic GABAA receptors, localized away from the synapses, are persistently exposed to a low concentration of ambient GABA. In order to detect such low concentrations of GABA for prolonged periods of time, extrasynaptic GABAA receptors should have a high affinity for GABA and should exhibit a low desensitization rate (Semyanov et al. 2004; Farrant & Nusser, 2005).
Tonic inhibition was first described in cerebellar granule cells of juvenile rats by Kaneda et al. (1995). These authors found that application of GABAA receptor antagonists not only blocked spontaneous IPSCs but also reduced the current required to keep the cell at a fixed potential, an effect that was associated with a decrease in the background noise. More recently, GABAA-mediated tonic conductance has been characterized in granule cells of the dentate gyrus (Nusser & Mody, 2002), in thalamo-cortical relay neurons of the ventral basal complex (Porcello et al. 2003), in adult hippocampal interneurons (Semyanov et al. 2003) and in hippocampal neurons in culture (Petrini et al. 2004).
In the perinatal period, the tonic activation of GABAA receptors by GABA present in the extracellular space, has been shown to occur prior to synapse formation (Valeyev et al. 1993; LoTurco et al. 1995; Owens et al. 1999; Demarque et al. 2002). This issue is of particular interest because of the role played by GABA during neuronal maturation (Owens & Kriegstein, 2002). In the immature hippocampus, GABA exerts a depolarizing action (Cherubini et al. 1991; Ben-Ari, 2002), which promotes a rise in intracellular calcium concentration via voltage-dependent calcium channels and NMDA receptors (Leinekugel et al. 1997). This may favour the development of new synapses and the formation of the adult neuronal circuit (Kasyanov et al. 2004).
Here a tonic activation of GABAA receptors was identified in CA1 and CA3 pyramidal cells but not in GABAergic interneurons of the neonatal hippocampus. This conductance appeared to be mediated by α5, β2/β3 and γ2 GABAA receptor subtypes. Blocking the tonic conductance with picrotoxin decreased membrane excitability and the glutamatergic drive of principal cells. These results suggest that the ambient GABA level, by enhancing cell excitability and synaptic activity, exerts a facilitatory role on the generation of giant depolarizing potentials (GDPs).
Methods
Slice preparation
Experiments were performed on hippocampal slices obtained from Wistar rats at postnatal day (P) 2 to P6 or at 7–8 weeks, as previously described (Gasparini et al. 2000). All experiments were carried out in accordance with the European Community Council Directive of November 24, 1986 (86/609EEC) and were approved by the local authority veterinary service. Briefly, animals were decapitated after being anaesthetized with an i.p. injection of urethane (2 g kg−1). The brain was quickly removed from the skull and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing (mm): NaCl 130, KCl 3.5, NaH2PO4 1.2, NaHCO3 25, MgCl2 1.3, CaCl2 2 and glucose 25; saturated with 95% O2–5% CO2 (pH 7.3–7.4). Transverse hippocampal slices (400 μm thick) were obtained by cutting with a vibratome and then stored at room temperature (22–24°C)in a holding bath containing the same ACSF as above. After a recovery period of at least 1 h, an individual slice was transferred to the recording chamber where it was continuously superfused with oxygenated ACSF at a rate of 2–3 ml min−1 at 33–34°C.
Electrophysiological recordings
Electrophysiological experiments were performed in CA1 and CA3 pyramidal cells, and in GABAergic interneurons localized in stratum radiatum using patch-clamp recordings in voltage-clamp mode. Pyramidal cells and interneurons were identified under infrared differential interference contrast microscopy according to their morphology, localization and their distinctive firing patterns. Six pyramidal cells and eight interneurons were injected with 3–4% biocytin (N-biotinyl-l-lysine, Sigma) for later morphological identification. Bipolar twisted NiCr-insulated electrodes were used to evoke synaptic responses in CA1 principal cells or in stratum radiatum interneurons.
Patch electrodes were pulled from borosilicate glass capillaries (Hingelberg, Malsfeld, Germany). They had a resistance of 5–7 MΩ when filled with an intracellular solution containing (mm): CsCl 137, Hepes 10, BAPTA 11, MgATP 2, MgCl2 2, CaCl2 1; pH adjusted to 7.3 with CsOH. In some experiments, in order to detect the firing rate of pyramidal cells and interneurons, 137 mm CsCl was replaced by 137 mm KCl.
In a subset of experiments, in order to record simultaneously GABAergic and glutamatergic currents we used a low-chloride solution containing (mm): potassium gluconate 135, KCl 20, Hepes 10, MgATP 4, and EGTA 0.5; pH adjusted to 7.2 with KOH. With this solution, the estimated chloride reversal potential was −50 mV. When required, gramicidin-perforated patch experiments were performed using the same intracellular solution plus 80 μg ml−1 gramicidin D (Sigma, Milan, Italy). A 50 mg ml−1 stock of gramicidin in dimethylsulphoxide (DMSO) was prepared freshly (< 2 h before recording) and sonicated. This was diluted with a gramicidin-free solution, sonicated again for 20–30 s, and centrifuged. Patch pipettes were back-filled with a gramicidin-containing solution and then the tip of the pipette was dipped into and filled with a gramicidin-free solution by applying a negative pressure for 20–30 s to facilitate cell-attached formation (seal resistance, > 3 GΩ). After around 40 min, series resistance decreased and stabilized at around 30 MΩ.
Recordings were made with a patch-clamp amplifier (Multiclamp 700A; Axon Instruments, Union City, CA, USA). The stability of the patch was checked by repeatedly monitoring the input and series resistance during the experiment. Cells exhibiting changes of 15–20% were excluded from the analysis. Additional experiments were performed in cell-attached mode. In these cases pipettes were filled with ACSF.
In another set of experiments, we tested the effect of picrotoxin on the amplitude of the field EPSPs evoked in the CA1 hippocampal region by stimulation of the Schaffer collaterals in stratum radiatum (at 0.1 Hz). Field EPSPs were recorded with glass microelectrode filled with an extracellular solution containing CGP 55845 (1 μm) to block GABAB receptors.
The drugs used were d-(−)-2-amino-5-phosphono-pentaoic acid, 6,7-dinitro-quinoxaline-2,3-dione (DNQX), CGP 55845, picrotoxin, SR 95531 (gabazine), L-655,708, etomidate (ETMD) and allotetrahydrodeoxycorticosterone (THDOC), all purchased from Tocris Cookson Ltd, Bristol, UK. NO-711 and strychnine were purchased from Sigma (Milan, Italy). Flurazepam was a gift from Dr S. Vicini (Georgetown University, Washington, DC, USA). All drugs were dissolved in DMSO, except gabazine, flurazepam, strychnine and NO-711 which were dissolved in water and picrotoxin which was dissolved in ethanol. The final concentration of DMSO in the bathing solution was 0.1%. At this concentration, DMSO alone did not modify the shape or the kinetics of synaptic currents. Drugs were applied in the bath via a three-way tap system, by changing the superfusion solution to the same soltion with the addition of drug(s). The ratio of flow rate to bath volume ensured complete exchange within 2 min.
Data acquisition and analysis
Data were sampled at 30 kHz with an A/D converter (Digidata 1322, Axon Instruments), filtered with a cut-off frequency of 1 kHz and analysed off-line with Clampfit 9.2 (Axon Instruments).
The amplitude of the tonic current was estimated by the outward shift of the baseline current after the application of the GABAA receptor antagonist picrotoxin (100 μm). Four epochs of 300–500 ms each were pooled to calculate the baseline current amplitude and its standard deviation. The resulting all-point histogram was fitted with a Gaussian function. Only current recordings that exhibited a stable baseline were included in the analysis. During the experiments in the whole-cell configuration, spontaneous GABAA-mediated postsynaptic currents were recorded from the holding potential of −60 mV. In particular, depending on the presence or absence of TTX, spontaneous miniature or action potential-dependent synaptic currents were identified. Synaptic currents were analysed with the AxoGraph 4.9 program (Axon Instruments). This program uses a detection algorithm based on a sliding template. The template did not induce any bias in the sampling of events because it was moved along the data trace by one point at a time and was optimally scaled to fit the data at each position. The detection criterion was calculated from the template-scaling factor and from how closely the scaled template fitted the data. The threshold for detection was set at 3.5 times the s.d. of the baseline noise. The decay phase of spontaneous synaptic currents was fitted with a single exponential.
Values are given as means ±s.e.m. Significance of differences was assessed using the paired or unpaired Student's t test or Wilcoxon matched-pair test as appropriate. P < 0.05 was considered significant.
Results
A tonic GABAA-mediated conductance is present in CA1 and CA3 principal cells
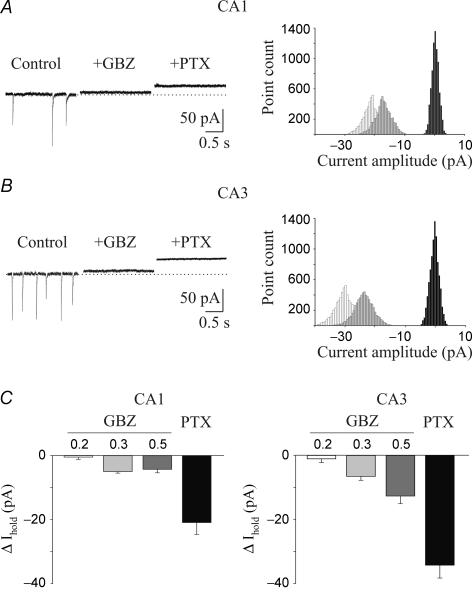
Whole-cell patch-clamp recordings in voltage-clamp mode were performed from 70 CA1 and 67 CA3 pyramidal cells in hippocampal slices obtained from rats at P2–P6. The majority of the experiments was performed in the presence of DNQX (20 μm) and CGP 55845 (1 μm) to block ionotropic glutamatergic AMPA/kainate and GABAB receptors, respectively. Principal cells had a resting membrane potential ranging from −48 to −66 mV (mean, −53.8 ± 2.1 mV) and a mean input resistance of 458 ± 55 MΩ (n = 9; see also Tyzio et al. 2003). They fired a few action potentials in response to long (800 ms) depolarizing current pulses, which rapidly accommodated. GABAA receptor-mediated tonic conductance was estimated by the shift in the baseline current obtained by blocking GABAA receptors with selective GABAA receptor antagonists. In the first set of experiments, in order to identify the contribution of action potential-dependent vesicular release of GABA from neighbouring synapses, we used low concentrations of gabazine. A concentration of 0.5 μm has been reported to selectively abolish spontaneously GABAA-mediated synaptic currents but not tonic currents (Bai et al. 2001; Semyanov et al. 2003). Spontaneous action potential-dependent and -independent synaptic currents occurred at the frequency of 3.9 ± 0.2 and 5.5 ± 0.3 Hz in CA1 (n = 18) and in CA3 (n = 17) pyramidal cells, respectively. Gabazine (0.5 μm) completely abolished spontaneous synaptic events (Fig. 1A and B) and produced an outward shift of the baseline current of 4.3 ± 1.1 and 12.7 ± 2.4 pA in CA1 and CA3 pyramidal cells, respectively (P < 0.01; Fig. 1C). The higher value observed in CA3 pyramidal cells may be attributed to the fact that, early in postnatal development, the major input to these cells (mossy fibres) is mainly GABAergic (Safiulina et al. 2006). Bath application of 0.3 μm gabazine reduced the amplitude and the frequency of spontaneous GABAergic events recorded in CA1 principal cells to 61% and 62%, respectively (n = 6). Similar results were obtained in CA3 pyramidal neurons (amplitude, 62%; frequency, 63%, n = 6). However, this effect was associated with a small outward shift in the baseline current (5 ± 0.5 and 6.6 ± 1.2 pA in CA1 and CA3 pyramidal cells, respectively). A lower concentration of gabazine (0.2 μm) decreased the frequency and the amplitude of spontaneous GABAA-mediated synaptic events in both CA1 (amplitude, 61%; frequency, 58%, n = 5) and CA3 pyramidal cells (amplitude, 62%; frequency, 57%, n = 6) with a negligible shift in the baseline current (0.5 ± 0.7 and 1.02 ± 1.14 pA in CA1 and CA3 principal cells, respectively). Summary data for gabazine-induced changes in tonic conductance are illustrated in Fig. 1C.
Figure 1. A tonic GABAA-mediated current is present in CA1 and CA3 pyramidal cells.
A, left, representative tracings of spontaneous GABAA-mediated synaptic currents recorded from a CA1 principal cell in control conditions and in the presence of gabazine (GBZ, 0.5 μm) and gabazine plus picrotoxin (PTX, 100 μm). Holding potential, −60 mV. Right, all-point histograms of 500 ms traces from the cell recorded on the left. Control, light grey; gabazine, dark grey; gabazine plus picrotoxin, black. B, as in A, but from a CA3 pyramidal cell. C, summary data of tonic currents obtained from 14 CA1 and 14 CA3 pyramidal cells in the presence of different concentrations (0.2, 0.3 and 0.5 μm) of gabazine and 100 μm of picrotoxin. In this and in the following figures the error bars indicate s.e.m.
To completely block GABAA-mediated conductance we used picrotoxin, which in a previous study in the immature hippocampus was shown to have a stronger inhibitory effect on tonic current than gabazine and bicuculline (Sipila et al. 2005). Addition of picrotoxin (100 μm) to gabazine produced a further outward shift in the holding current that was more pronounced in CA3 than CA1 principal cells (Fig. 1). On average, this was 20.9 ± 3.7 pA in CA1 (n = 9) and 34.2 ± 4.0 pA in CA3 (n = 8). Current density (tonic current/capacitance) values were 0.57 ± 0.1 and 1.03 ± 0.1 pA pF−1 in CA1 (n = 6) and CA3 (n = 6) pyramidal cells, respectively. These values were significantly different (P < 0.005).
As picrotoxin can also block glycine receptors (Lynch, 2004), which are abundantly expressed in both the immature (Ito & Cherubini, 1991) and the adult hippocampus (Song et al. 2006), in the next series of experiments we investigated whether the ambient glycine level contributes to the picrotoxin-sensitive tonic conductance. To this aim we applied 0.5 μm strychnine, which at this concentration is known to preferentially block glycine receptors (Ito & Cherubini, 1991). In the presence of strychnine, no significant changes in the holding current were observed (CA1, 0.5 ± 0.3 pA; CA3, 1.1 ± 0.2 pA, n = 5 for both). This allows the contribution of glycine to picrotoxin-sensitive tonic conductance to be excluded.
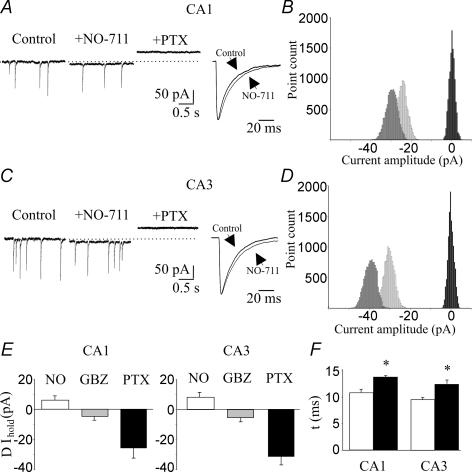
To test the effect of ambient GABA levels on GABA transporters, we applied NO-711, which selectively blocks the neuronal and glial GABA transporter GAT-1 (Borden, 1996,Semyanov et al. 2003; Petrini et al. 2004). NO-711 (10 μm) produced a small inward shift of the baseline current (CA1, 6.3 ± 2.8 pA, n = 6; CA3, 8.6 ± 3.3 pA, n = 7; Fig. 2) and slowed down the decay kinetics of spontaneous GABAA-mediated synaptic events (the mean time constant (τmean): CA1, increased from 13.1 ± 0.6 to 16.7 ± 0.5 ms, P < 0.05; CA3, from 13.5 ± 0.4 to 15.7 ± 0.7 ms, P < 0.05; Fig. 2A, C and F). Consistent with a higher concentration of ambient GABA in the presence of NO-711, the shift in the baseline currents produced by picrotoxin was larger (CA1, 31.4 ± 4.5 pA; CA3, 39.8 ± 4.3 pA; Fig. 2E). These values represent the sum of the shift produced by NO-711 plus picrotoxin. In CA1 pyramidal cells, the mean baseline shift induced by picrotoxin in the presence of NO-711 was significantly different from control (P < 0.05); in CA3 it did not reach a significant level (P = 0.2). These data indicate that the uptake of GABA from principal cells and astrocytes contributes to the clearance of this neurotransmitter from the extracellular space.
Figure 2. Contribution of the GABA transporter GAT-1 to tonic GABAA-mediated conductance in CA1 and CA3 pyramidal cells.
A, left, representative traces of spontaneous GABAA-mediated currents recorded from a CA1 principal cell in control conditions, during application of the GAT-1 blocker NO-711 (10 μm) and NO-711 plus picrotoxin. Right, spontaneous events obtained in control conditions and in presence of NO-711, normalized and superimposed on the right (each trace is the average of 40–50 events). B, all-point histogram of 500 ms traces from the cell recorded on the left in control conditions (light grey), in the presence of NO-711 (dark grey) and NO-711 plus picrotoxin (black). C and D, as in A and B, but from a CA3 principal cell. E, summary data obtained from six CA1 and seven CA3 principal cells. F, decay kinetics (τ) of spontaneous events detected in control conditions (open columns), and in the presence of NO-711 (filled columns) from six CA1 and seven CA3 pyramidal cells. *P < 0.05.
To assess further the role of network activity in tonic GABAA-mediated conductance, additional experiments were performed in the presence of TTX (1 μm), which blocks voltage-activated sodium channels and propagated action potentials. In these conditions, spontaneous miniature GABAA-mediated synaptic currents, occurring at low frequency (CA1, 1.9 ± 0.6 Hz, n = 5; CA3, 2.6 ± 0.5 Hz, n = 8) were recorded. Application of gabazine (0.5 μm) blocked miniature events and caused only a negligible shift in the holding current (see Fig. 3). Further addition of picrotoxin (100 μm) induced an outward shift of the baseline current of 11.2 ± 2.0 and 24.1 ± 2.5 pA in CA1 and CA3 principal cells, respectively (Fig. 3). These values were significantly different (P < 0.05) from those obtained in the absence of TTX and indicate that action potential-dependent release of GABA contributes to 46% and 29.5% of the magnitude of the total currents observed in CA1 and CA3 pyramidal cells, respectively.
Figure 3. The network activity contributes to GABAA-mediated tonic conductance in pyramidal cells.
A, left, representative traces of spontaneous GABAA-mediated miniature synaptic currents recorded from a CA1 pyramidal cell in the presence of TTX (1 μm) alone (control), and during bath application of gabazine (GBZ) and gabazine plus picrotoxin. Right, all-point histograms of 500 ms traces recorded from the cell shown on the left. Control, white; gabazine, grey; gabazine plus picrotoxin, black. B, as in A, but from a CA3 principal cell. C, summary data of tonic currents obtained from five CA1 and eight CA3 pyramidal cells recorded in the presence of TTX.
Stratum radiatum interneurons do not bear a tonic GABAA-mediated conductance
A previous study in adult guinea-pig hippocampus has revealed that in the presence of an intact GABA uptake system, picrotoxin does not significantly affect the holding current of CA1 pyramidal cells but produces a significant shift of the baseline current in stratum radiatum GABAergic interneurons (Semyanov et al. 2003). Therefore, in the following experiments we explored whether a similar GABAA-mediated tonic conductance could be detected in immature GABAergic interneurons. This may be relevant for GABAergic signalling during postnatal development. Stratum radiatum interneurons had a mean resting membrane potential of −53 ± 1.7 mV and a mean input resistance of 709 ± 53 MΩ (n = 9). They discharged at high frequency (20.7 ± 2.1 Hz, n = 12) in response to long (800 ms) depolarizing current pulses. Biocytin injection revealed the typical shape of interneurons (n = 8; data not shown). At the holding potential of −60 mV, spontaneous inwardly directed synaptic currents occurring at 1.7 ± 0.3 Hz (n = 11) were recorded. They were GABAergic in origin, as they were readily blocked by gabazine (0.5 μm; data not shown). Gabazine did not modify the holding current. Tonic conductance was measured in 18 CA1 and 17 CA3 stratum radiatum interneurons. Addition of picrotoxin (100 μm) produced a very small outward shift of the baseline current. On average this was 4.4 ± 1.4 and 5.9 ± 2.7 pA for CA1 and CA3 stratum radiatum interneurons, respectively. These values were not significantly different (P > 0.05) and therefore data obtained from this set of experiments were pooled (Fig. 4C). The small picrotoxin-induced shift in holding current could be related to the different size of interneurons with respect to principal cells. However, this is unlikely, because cell capacitance in interneurons was similar to that found in CA1 pyramidal cells (interneurons, 32.2 ± 1.4 pF; CA1 principal cells, 36 ± 1.7 pF).
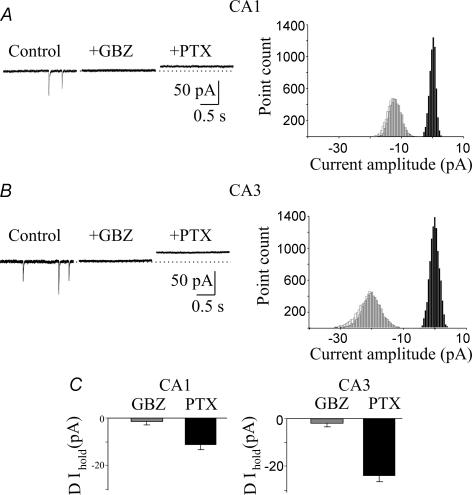
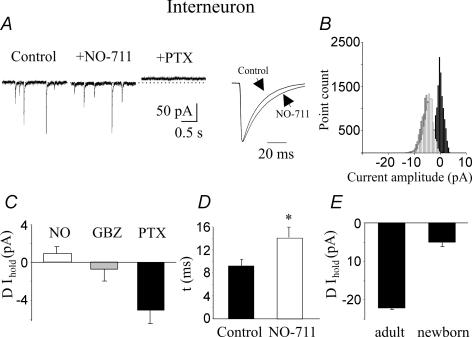
Figure 4. Lack of a sustained tonic GABAA-mediated conductance in stratum radiatum interneurons.
A, left, representative traces of spontaneous GABAA-mediated currents obtained from stratum radiatum interneurons in control conditions, during application of NO-711 (10 μm) and NO-711 plus picrotoxin. Right, spontaneous events obtained in control conditions and in the presence of NO-711, normalized and superimposed (each trace is an average of 50 responses). B, all-point histograms of 500 ms traces recorded on the left. C, summary data obtained from 19 interneurons. D, mean deactivation kinetic values (τ, of spontaneous events recorded in control conditions (n = 9) and in the presence of NO-711; n = 11; *P < 0.05). E, average values of tonic GABAA-mediated conductance recorded from stratum radiatum interneurons in slices obtained from adult (n = 4) and newborn (n = 16) animals.
To see whether the small change in baseline current observed in stratum radiatum interneurons is related to GABA clearance from the extracellular space via a very efficient uptake system, picrotoxin was applied in the presence of the GAT-1 antagonist NO-711. NO-711 slowed down the decay kinetics of spontaneous synaptic events (τmean increased from 7.9 ± 0.6 to 10.1 ± 0.9 ms, P < 0.05; Fig. 4A and D) without modifying significantly the holding currents (0.9 ± 0.8 pA, n = 19; Fig. 4). Therefore, the lack of a tonic inhibition in interneurons cannot be attributed to an active uptake system, which maintains the extracellular GABA concentration at very low levels. To see whether the lack of tonic GABAA-mediated conductance in interneurons is a transient phenomenon, restricted to postnatal development, we measured tonic GABAA-mediated conductance in stratum radiatum interneurons recorded from hippocampal slices obtained from 7- to 8-week-old animals (n = 4). In agreement with the results of Semyanov et al. (2003), picrotoxin induced a clear shift in the baseline current of 22 ± 0.4 pA (Fig. 4E). Therefore, it seems likely that, in the immediate postnatal period, the amount of ambient GABA sensed by interneurons is negligible.
Extrasynaptic GABAA receptors involved in the tonic conductance in principal cells
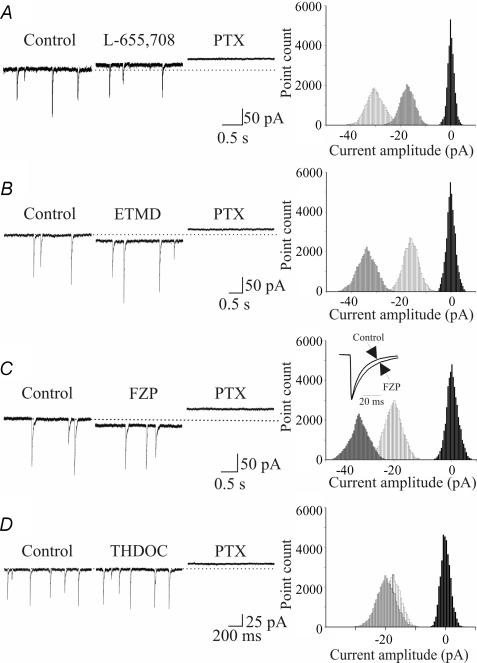
To identify the extrasynaptic GABA receptor subtypes involved in tonic GABAA-mediated conductance detected in principal cells, we tested several substances known to interact with GABAA receptors in a subunit-selective manner. Firstly, we applied L-655,708, an inverse GABAA receptor agonist, which is at least 50-fold more selective for α5β2γ2 GABAA receptors than for heteromeric receptors containing the α1, α2 and α3 subunits (Quirk et al. 1996; Casula et al. 2001). As shown in the representative example of Fig. 5A, L-655,708 (5 μm) produced a small outward shift of the holding current (CA1, 14.4 ± 2.4 pA, n = 6; CA3, 13.1 ± 3.5 pA, n = 5). Similar results were obtained with L-655,708 (30 μm) which produced a baseline shift of 15.7 ± 2.6 pA in CA1 and 18.9 ± 4.7 pA in CA3 pyramidal cells (data not show). Because α5 subunits frequently co-assemble with β3 subunits in pyramidal cells (Mertens et al. 1993; Sur et al. 1998) and these subunits are expressed in extrasynaptic areas of the hippocampus (Pirker et al. 2000), we tested the sensitivity of the tonic conductance to ETMD, a compound known to be a positive allosteric modulator of receptors containing the β2 and β3 subunits (Sanna et al. 1997; Hill-Venning et al. 1997). ETMD, at 100 nm, produced an inward shift of the baseline current (CA1, 17.7 ± 4.8 pA, n = 8; CA3, 19.9 ± 3.6 pA, n = 10; Fig. 5B), revealing the presence of β2/β3 subunits. The contribution of γ2 subunits was tested with flurazepam, a positive allosteric modulator of γ2-containing receptors which are abundantly expressed at extrasynaptic sites (Christie et al. 2002; Danglot et al. 2003; Petrini et al. 2004). Flurazepam, at 3 μm, induced an inward shift in the baseline current of 6.1 ± 0.7 pA in CA1 (n = 8) and 11.9 ± 0.9 pA in CA3 principal cells (n = 7). A higher concentration of flurazepam (10 μm) produced an inward shift in the baseline currents of 14.8 ± 2.9 pA in CA1 (n = 6) and 21.8 ± 3.7 pA in CA3 principal cells (n = 5; Fig. 5C). This effect was associated with a slowing of the decay kinetics of spontaneous GABAA-mediated synaptic currents (CA1, from 17.3 ± 1.5 to 26 ± 0.4 ms, P < 0.05; CA3, from 16.9 ± 1.8 to 24.8 ± 1.2 ms P < 0.05; see inset in Fig. 5C).
Figure 5. Different extrasynaptic GABAA receptor subunits mediate tonic currents in CA1 principal cells.
Left, inward and outward shifts in the holding current obtained in control conditions and in the presence of the α5 inverse agonist L-655,708 (5 μm, A), the β2/β3 enhancer ETMD (100 nm, B), the γ2 positive allosteric modulator flurazepam (FZP, 10 μm, C) and the δ antagonist THDOC (10 nm, D). Right, related all-point histograms for the traces shown on the left. The inset in C represent spontaneous events obtained in control conditions and in the presence of FZP, normalized and superimposed (each trace is the average of 60 responses).
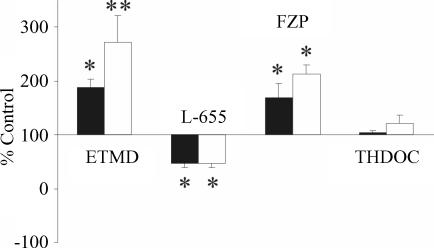
The tonic conductance was almost unaffected by THDOC (10 nm), a δ subunit selective neuroactive steroid (Korpi & Luddens, 1997; Wall, 2002; Korpi et al. 2002; Fig. 5D). THDOC produced an inward shift of the baseline current of 1.1 ± 0.9 and 2.3 ± 1.5 pA, in CA1 (n = 6) and CA3 (n = 6) pyramidal cells, respectively, suggesting that δ subunits are poorly expressed on principal cells. Data for different agonists and antagonists presented as percentage control relative to picrotoxin-induced shift in current amplitude for 27 CA1 and 26 CA3 principal cells are summarized in Fig. 6. All drugs except THDOC changed tonic conductance in a significant manner. However, no significant differences in the effect of drugs between CA1 and CA3 pyramidal cells were observed.
Figure 6. Pharmacological characterization of GABAA receptor subunits contributing to the tonic GABAA-mediated conductance recorded in CA1 (filled columns) and CA3 (open columns) pyramidal cells.
Data are presented as percentage control relative to the PTX-mediated shift in current amplitude (Wilcoxon matched-pair test, *P < 0.05; **P < 0.01). Etomidate (ETMD) (n = 8), L-655,708 (L-655, n = 6), flurazepam (FZP, n = 6) and allotetrahydrodeoxycorticosterone (THDOC) (n = 6).
Tonic conductance increases excitability and glutamatergic synaptic signalling in pyramidal cells but not in interneurons
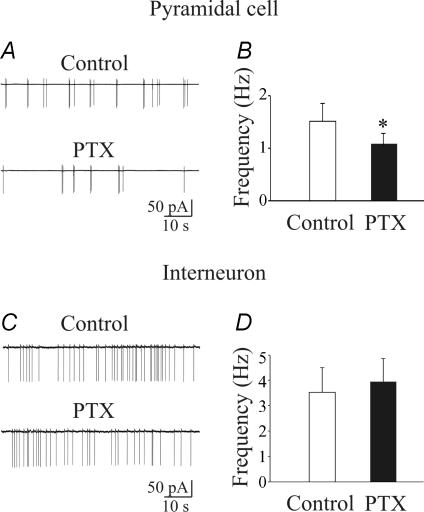
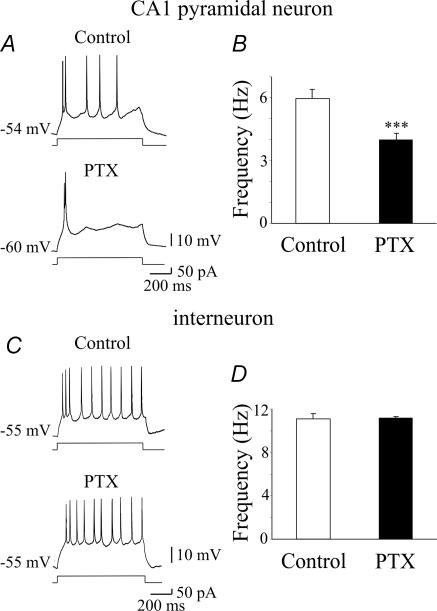
As already mentioned, early in postnatal life GABA exerts a depolarizing and excitatory action (Cherubini et al. 1991; Ben-Ari, 2002), which may change GABAergic signalling at the network level. Therefore, experiments were undertaken to assess whether blocking a GABAA-mediated tonic conductance with picrotoxin would alter the activity of CA1 pyramidal cells and stratum radiatum interneurons. To this purpose, we used cell-attached recordings, which do not modify the intracellular milieu of the cells. As illustrated in Fig. 7, whereas picrotoxin (100 μm) reduced the spontaneous firing of CA1 principal cells (from 1.5 ± 0.4 to 1.1 ± 0.3 Hz; P < 0.05; n = 6), it did not affect the firing of interneurons (3.6 ± 1.3 and 3.9 ± 1.2 Hz before and during application of picrotoxin, respectively; P > 0.5; n = 7). Consistent with whole-cell data, these results suggest that the level of ambient GABA exerts a powerful excitatory action on principal cells enhancing their firing, whereas it does not affect the activity of stratum radiatum interneurons. In additional experiments, the functional role of tonic GABAA-mediated conductance on membrane excitability of CA1 principal cells and interneurons was assessed using gramicidin-perforated patch, which allows the anionic conditions of the cell to be preserved (Kyrozis & Reichling, 1995). Steady depolarizing and hyperpolarizing current pulses of different intensities lasting 800 ms were delivered to CA1 principal cells and interneurons to measure the firing properties and the membrane input resistance of the cells under different experimental conditions. Depolarizing current pulses elicited in principal cells and GABAergic interneurons electrotonic potentials that after reaching a certain threshold gave rise to action potentials (see controls in Fig. 8). Action potential firing accommodated in principal cells, whereas in interneurons it was usually maintained throughout the duration of the current pulse. Bath application of picrotoxin (100 μm), induced in CA1 pyramidal cells a membrane hyperpolarization which ranged from 2 to 10 mV (4.4 ± 2.8 mV, n = 12; see also Ben-Ari et al. 1989; Sipila et al. 2005) and was associated with a 9.3 ± 1.4% decrease in membrane input resistance. At the peak of picrotoxin-induced hyperpolarization, depolarizing current pulses (of the same amplitude as the controls) caused a 33.4 ± 8.6% reduction (from 6.0 ± 0.4 to 4.0 ± 0.3; P < 0.001) in the number of spikes (Fig. 8A and B). A similar (5–10 mV) membrane hyperpolarization, induced by injecting a hyperpolarizing current through the patch pipette, in the absence of picrotoxin, produced a significant reduction (32.9 ± 7.2%; n = 6; P < 0.01; data not shown) in the number of spikes. This value was not significantly different from that obtained in the presence of picrotoxin (P > 0.5). These results indicate that picrotoxin-induced reduction in cell excitability can be mainly attributed to membrane hyperpolarization which enhances the threshold for spike generation.
Figure 7. Ambient GABA enhances spontaneous firing in CA1 principal cells but not in stratum radiatum interneurons.
A, example trace recorded in cell-attached mode from a CA1 pyramidal cell (postnatal day 4) in control conditions and in the presence of picrotoxin. B, each column represents the mean firing frequency obtained from six CA1 principal cells in control conditions and during bath application of picrotoxin (*P < 0.05). C and D, as in A and B, but for stratum radiatum interneurons (n = 7).
Figure 8. Ambient GABA enhances pyramidal cell excitability.
A, individual traces showing the firing patterns of a CA1 pyramidal cell in response to a steady depolarizing current pulse, before (upper) and during (lower) bath application of picrotoxin (PTX, 100 μm). In this cell, PTX induced a 6 mV membrane hyperpolarization. B, changes in firing frequency obtained by depolarizing CA1 principal cells in control conditions and during bath application of PTX. The firing rate was tested at the peak of PTX-induced membrane hyperpolarization (ranging in different cells from 2 to 10 mV, n = 12, ***P < 0.001). C and D, as in A and B but for stratum radiatum interneurons (n = 4, P > 0.5).
Similar experiments performed on stratum radiatum interneurons failed to reveal any picrotoxin-induced change in membrane potential or in firing rate (spike frequency was 11.1 ± 0.5 and 11.2 ± 0.1 Hz, before and during application of picrotoxin, respectively, n = 4, P > 0.5; Fig. 8C and D). Picrotoxin did not modify the membrane input resistance of these cells.
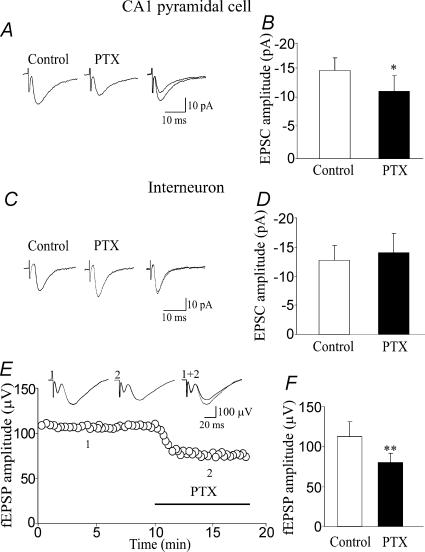
In additional experiments, the ability of picrotoxin to modify the amplitude of the EPSCs or EPSPs was tested in CA1 pyramidal cells recorded in the whole-cell configuration with a low-Cl− intrapipette solution (chloride reversal potential, −50 mV, see Methods). Picrotoxin (100 μm) reduced the amplitude of the EPSCs evoked in CA1 pyramidal cells (held at −45 to −50 mV) by stimulation of the Schaffer collateral (from 15.2 ± 2.2 to 11.7 ± 2.7 pA, n = 6, P < 0.05; Fig. 9A and B). In all these experiments, addition of DNQX (20 μm) completely abolished the EPSCs (data not shown). In three experiments performed in current-clamp mode, the EPSPs reached the threshold for action potentials. In these cases, picrotoxin (100 μm) abolished the action potentials indicating that tonic GABAA-mediated conductance may bring cells to fire (data not shown). In similar experiments performed from CA1 stratum radiatum interneurons (n = 7), picrotoxin (100 μm) did not produce any significant change in amplitude of the EPSCs (the amplitude of EPSCs was 12.7 ± 2.6 and 14.1 ± 3.3 pA before and after application of picrotoxin, respectively, P > 0.5; Fig. 9C and D).
Figure 9. A tonic GABAA-mediated conductance enhances the glutamatergic drive to principal cells.
A, synaptic currents evoked at −50 mV in a CA1 pyramidal neuron by stimulation of the Schaffer collateral, before (thin line) and during (thick line) application of PTX. On the right the two currents are superimposed (each trace is the average of 15 responses). B, each column represents the mean amplitude of EPSCs evoked in CA1 principal cells by stimulation of the Schaffer collateral in control (open column) and during bath application of PTX (filled column, n = 6; *P < 0.05). C and D, as for A and C, but for EPSCs evoked in stratum radiatum interneurons (n = 7). Note that PTX failed to reduce the amplitude of EPSCs in interneurons. E, in the graph the amplitude of fEPSPs evoked by Schaffer collateral stimulation before and during (indicated by horizontal bar) application of PTX (100 μm) is plotted against time. Each point represents the mean of three responses. In the inset, fEPSPs recorded at the time indicated (each trace is the average of three responses). The reduction in amplitude of the fEPSP was associated with a small decrease in the amplitude of the afferent volley (12%). F, each column represents the mean amplitude of fEPSPs evoked by stimulation of the Schaffer collateral in control (open column) and during bath application of PTX (filled column, n = 9 slices from nine P3–P5 rats; **P < 0.01).
To further investigate whether a tonic GABAA-mediated conductance contributes to glutamatergic signalling, the effect of picrotoxin was tested on field EPSPs (fEPSPs) evoked in the CA1 region by stimulation of the Schaffer collateral (n = 9). Picrotoxin (100 μm) caused a significant decrease in amplitude of the fEPSPs (from 112 ± 19 to 79 ± 12 μV, P < 0.01; Fig. 9E and F). In three cases where the presynaptic afferent volley was clearly measurable, the change in amplitude of the fEPSP was associated with a small reduction in the amplitude of the afferent volley (from 41 ± 8 to 36 ± 12 μV). All together, these data suggest that picrotoxin may also block presynaptic GABAA receptors thus directly affecting glutamate release.
Discussion
The results of the present study shows that, during the first week of postnatal life, a tonic GABAA-mediated conductance is present in CA1 and CA3 principal cells but not in GABAergic interneurons localized in stratum radiatum. This conductance is at least in part activated at the network level by spillover of GABA from adjacent synapses as demonstrated by the experiments with TTX. Blocking the clearance of GABA with NO-711 slightly enhances the tonic conductance in principal cells but not in GABAergic interneurons. This cell-type specificity of GABA conductance would contribute to the enhancement of cell excitability in principal cells thus exerting a facilitatory role on generation of GDPs at the network level.
A tonic GABAA-mediated conductance is present in CA1 and CA3 principal cells but not in stratum radiatum interneurons
Previous data from the hippocampus of embryonic and newborn animals have indicated that GABAA receptors develop before the generation of conventional synapses (Demarque et al. 2002). According to Tyzio et al. (1999), at birth, a large number of CA1 pyramidal neurons are silent because they lack synaptic connections. However, these neurons exhibited a tonic conductance generated by GABA released in a non-conventional way (Demarque et al. 2002). In the present study in animals at P2–P6, silent neurons were only rarely observed and we focused only on those cells expressing spontaneous synaptic events. In contrast with previous reports from the adult hippocampus or cultured hippocampal neurons (Bai et al. 2001; Semyanov et al. 2003), blocking synaptic currents with 0.3 or 0.5 μm gabazine produced a small shift in the holding current, suggesting the involvement of both synaptic and extrasynaptic receptors. This apparent discrepancy could be explained by a different sensitivity to gabazine of extrasynaptic GABAA receptors present in the neonatal rat hippocampus (see also Sipila et al. 2005). In addition, we cannot exclude the possibility that the lower sensitivity of extrasynaptic GABAA receptors to gabazine than synaptic receptors may be due to the different pharmacology of constitutive GABA-independent gating of tonically active GABAA receptors (McCartney et al. 2007).
The lack of baseline shift observed when gabazine was applied in the presence of TTX, suggests that GABA spillover from adjacent synapses needs the activity of the entire network. We do not know the exact concentration of GABA in the extracellular milieu. In a previous in vivo study, the concentration of GABA in the extracellular space was estimated to be 1 μm (Lerma et al. 1986). This low concentration of GABA is usually maintained by a very efficient uptake system (Borden, 1996), which actively takes up GABA into neurons and glia (Cherubini & Conti, 2001). Although at early developmental stages the functional role of GABA transporters has been questioned (Demarque et al. 2002), the NO-711-induced changes in decay kinetics of synaptic currents and in the holding current suggest that GAT-1 is present and functional already at P2. This is in agreement with the observation that in the neonatal hippocampus, GABA uptake limits the action of GABA during GDPs (Sipila et al. 2004) and controls GABAergic transmission at mossy fibre–CA3 synapses (Safiulina et al. 2006).
By contrast, application of picrotoxin to stratum radiatum interneurons produced only a very small shift in the outward currents indicating that, early in postnatal life, these cells can only ‘sense’ a limited amount of ambient GABA. In a previous study in adult guinea-pigs, it was demonstrated that tonic GABAA-mediated inhibition is more prominent in interneurons than in principal cells (Semyanov et al. 2003). Several hypotheses can be put forward to explain the present results. (i) The smaller size of interneurons with respect to principal cells. However, this is unlikely because similar values of cell capacitance were detected in interneurons and CA1 principal cells. (ii) The presence of a very efficient transport system, which maintains the extracellular GABA concentration at very low levels. This was not the case because, unlike in adult guinea-pigs (Semyanov et al. 2003), NO-711 failed to reveal a persistent tonic conductance in immature interneurons despite significant changes in decay kinetics of spontaneous synaptic events. However, we cannot exclude the involvement of GABA transporters other than GAT-1. (iii) The geometrical arrangement of pyramidal cells and interneurons may differentially restrict GABA diffusion and alter the local extracellular concentration of the neurotransmitter. (iv) It is also possible that in immature interneurons, extrasynaptic high-affinity GABAA receptors able to sense low concentrations of GABA are poorly expressed. Although this is an interesting issue, at present no data are available.
In agreement with other groups (Fritschy & Mohler, 1995; Sperk et al. 1997; Danglot et al. 2003; Wei et al. 2003), we found that extrasynaptic receptor subunits able to sense ambient GABA levels in principal cells are β2/β3 α5 and γ2 subtypes. Together with the δ, the α5 subunit, which is highly expressed in the neonatal rat hippocampus (Laurie et al. 1992; Didelon et al. 2000), is very sensitive to GABA and desensitizes slowly (Bai et al. 2001; Stell & Mody, 2002; Yeung et al. 2003). This subunit is primarily localized at extrasynaptic sites (Fritschy et al. 1998; Crestani et al. 2002; Brunig et al. 2002; Houser & Esclapez, 2003). The lack of a clear effect of THDOC on the baseline current of principal cells allows the involvement of δ subunits to be excluded, although in the dentate gyrus their contribution to tonic inhibition is well established (Overstreet & Westbrook, 2001; Nusser & Mody, 2002; Stell et al. 2003). The γ2 subunits are required for the observed facilitatory effect of flurazepam on tonic inhibition (Pritchett et al. 1989; Sigel et al. 1990; Petrini et al. 2004). However, it must be pointed out that benzodiazepine sensitivity may also be due to α5 subunit-containing receptors (Mertens et al. 1993; Caraiscos et al. 2004). On the basis of recent studies demonstrating that α5 may co-assemble with the β2/β3 subunits, we can speculate that in immature pyramidal neurons extrasynaptic native receptors mainly belong to α5β2γ2 or α5β3γ2 receptor types (see Sur et al. 1998; Crestani et al. 2002; Christie & De Blas, 2002; Caraiscos et al. 2004).
Functional role of a GABAA-mediated conductance in immature pyramidal cells
What could be the functional role of GABAA-mediated tonic conductance in immature pyramidal cells? In adult cerebellar granule cells, the ambient GABA level has been shown to regulate rate-code information defined as the relationship between the mean excitation and the mean output firing rate (Mitchell & Silver, 2003; Semyanov et al. 2004; Farrant & Nusser, 2005). According to the nature of excitatory inputs, blocking tonic inhibition produced either a shift to the right (Brickley et al. 1996; Hamann et al. 2002; Chadderton et al. 2004) or a reduction in the slope of the input–output curve (Mitchell & Silver, 2003). The latter would correspond to a reduction in the gain of function (Mitchell & Silver, 2003; Semyanov et al. 2004). In the adult hippocampus, selective blockade of GABAA receptors mediating the tonic conductance with a low concentration of picrotoxin was able to enhance the inhibitory drive to principal cells but not to GABAergic interneurons (Semyanov et al. 2003, 2004; Farrant & Nusser, 2005). In the neonatal rat hippocampus, because of the depolarizing and excitatory action of GABA (Cherubini et al. 1991; Ben-Ari, 2002), a persistent tonic conductance was found to enhance pyramidal cell excitability. Recently it has been proposed that during postnatal development GABA exerts a shunting inhibition on CA3 stratum lucidum interneurons (Banke & McBain, 2006). Therefore, blocking the shunting inhibition with picrotoxin should enhance cell firing in interneurons. The observation that in cell-attached experiments, picrotoxin was unable to modify the firing rate of interneurons suggests that not all synaptic inputs onto interneurons remained shunting throughout development. Thus, we cannot exclude the possibility that at the network level, the shunting inhibitory effect of GABA on some interneurons may be counterbalanced by its excitatory action on others. The reduction in the firing rate observed in CA1 principal cells upon removal of the tonic depolarizing action of GABA could be attributed to the enhanced threshold for action potential generation following picrotoxin-induced membrane hyperpolarization. In support of this view we observed that similar changes in action potential firing were obtained when the membrane was artificially hyperpolarized by injecting a steady current into the patch pipette in the absence of picrotoxin. Although we did not examine whether picrotoxin could modify cell excitability in CA3 pyramidal neurons, it is likely that similar changes occur also in this hippocampal area. However, in immature CA3 pyramidal cells, because of the partial GABAergic nature of the mossy fibres (Safiulina et al. 2006), the contribution of the phasic to the total GABAA-mediated conductance (blocked by picrotoxin) would be more important. In both CA3 and CA1 pyramidal cells, the enhanced firing rate would increase the release of glutamate into target neurons.
The depressing action of picrotoxin on the amplitude of Schaffer collateral-evoked EPSCs is surprising because synaptic currents were recorded at the reversal potential of GABA when no currents were flowing through the cell membrane. In line with field potential data, we can speculate that ambient GABA may activate presynaptic (axon or terminal) GABAA receptors present on glutamatergic terminals. By depolarizing glutamatergic terminals, GABA may enhance glutamate release in the same way as recently described by Jang et al. (2005). Although further experiments are needed to validate this hypothesis, it is clear from the present data that the enhancement of the EPSP amplitude by GABA would increase the temporal and spatial window over which signal integration may occur, making the occurrence of action potentials more probable and leading to an increased glutamatergic drive to interneurons. This would compensate for the reduced amount of glutamate released from principal cells in the perinatal period (Hosokawa et al. 1994; Tyzio et al. 1999; Demarque et al. 2002; Safiulina et al. 2005).
In conclusion, the present data clearly show that ambient GABA increases synaptic activity and cell firing within the hippocampal network. This would allow the threshold for generation of GDPs to be reached as recently demonstrated by Menendez de la Prida et al. (2006). Regarding this, it is worth noting that the interneuronal network is unable to generate GDPs in the absence of a glutamatergic drive (Bolea et al. 1999). Therefore, the present results strengthen even further the role of principal cells in network synchronization and generation of GDPs during postnatal developmental.
Acknowledgments
We are grateful to Majid H. Mohajerani and Maria Pytel for participating in some experiments and to Manuela Lough for carefully reading the manuscript. This work was supported by grants from Ministero Istruzione Universita′ e Ricerca (MIUR-PRIN 2005 to E.C.) and from the European Union (Project 503221 to E.C.). A.O. was supported by the Program for Training and Research in Italian Laboratories, International Centre for Theoretical Physics, Trieste, Italy.
References
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci. 2006;26:11720–11725. doi: 10.1523/JNEUROSCI.2887-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolea S, Avignone E, Berretta N, Sanchez-Andres JV, Cherubini E. Glutamate controls the induction of GABA-mediated giant depolarizing potentials through AMPA receptors in neonatal rat hippocampal slices. J Neurophysiol. 1999;81:2095–2102. doi: 10.1152/jn.1999.81.5.2095. [DOI] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cellular granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol. 2002;443:43–55. doi: 10.1002/cne.10102. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula MA, Bromidge FA, Pillai GV, Wingrove PB, Martin K, Maubach K, Seabrook GR, Whiting PJ, Hadingham KL. Identification of amino acid residues responsible for the alpha5 subunit binding selectivity of L-655,708, a benzodiazepine binding site ligand at the GABAA receptor. J Neurochem. 2001;77:445–451. doi: 10.1046/j.1471-4159.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D. Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biophys Mol Biol. 2005;87:3–16. doi: 10.1016/j.pbiomolbio.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M. Integration of quanta in cerebellar granule cells during sensory processing. Nature. 2004;428:856–860. doi: 10.1038/nature02442. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Conti F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24:155–162. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Christie SB, de Blas AL. Alpha5 subunit-containing GABAA receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Christie SB, Li RW, Miralles CP, Riquelme R, Yang BY, Charych E, Wendou Yu, Daniels SB, Cantino ME, De Blas AL. Synaptic and extrasynaptic GABAA receptor and gephyrin clusters. Prog Brain Res. 2002;136:157–180. doi: 10.1016/s0079-6123(02)36015-1. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABAA receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Bessis A. Association of gephyrin with synaptic and extrasynaptic GABAA receptors varies during development in cultured hippocampal neurons. Mol Cell Neurosci. 2003;23:264–278. doi: 10.1016/s1044-7431(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36:1051–1061. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- Didelon F, Mladinic' M, Cherubini E, Bradbury A. Early expression of GABAA receptor delta subunit in the neonatal rat hippocampus. J Neurosci Res. 2000;62:638–643. doi: 10.1002/1097-4547(20001201)62:5<638::AID-JNR3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Johnson DK, Mohler H, Rudolph U. Independent assembly and subcellular targeting of GABAA-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci Lett. 1998;249:99–102. doi: 10.1016/s0304-3940(98)00397-8. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Saviane C, Voronin L, Cherubini E. Silent synapses in the developing hippocampus: lack of functional AMPA receptors or low probability of glutamate release? Proc Natl Acad Sci U S A. 2000;97:9741–9746. doi: 10.1073/pnas.170032297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hill-Venning C, Belelli D, Peters JA, Lambert JJ. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. Br J Pharmacol. 1997;120:49–756. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa Y, Sciancalepore M, Stratta F, Martina M, Cherubini E. Developmental changes in spontaneous GABAA-mediated synaptic events in rat hippocampal CA3 neurons. Eur J Neurosci. 1994;6:805–813. doi: 10.1111/j.1460-9568.1994.tb00991.x. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Downregulation of the α5 subunit of the GABAA receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus. 2003;13:633–645. doi: 10.1002/hipo.10108. [DOI] [PubMed] [Google Scholar]
- Ito S, Cherubini E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. J Physiol. 1991;440:67–83. doi: 10.1113/jphysiol.1991.sp018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Ito Y, Akaike N. Feed-forward facilitation of glutamate release by presynaptic GABAA receptors. Neuroscience. 2005;135:737–748. doi: 10.1016/j.neuroscience.2005.06.030. [DOI] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol. 1995;485:419–435. doi: 10.1113/jphysiol.1995.sp020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABAA receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Luddens H. Furosemide interactions with brain GABAA receptors. Br J Pharmacol. 1997;120:741–748. doi: 10.1038/sj.bjp.0700922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- McCartney MR, Deeb TZ, Henderson TN, Hales TG. Tonically active GABAA receptors in hippocampal pyramidal neurons exhibit constitutive GABA-independent gating. Mol Pharmacol. 2007;71:539–348. doi: 10.1124/mol.106.028597. [DOI] [PubMed] [Google Scholar]
- Menendez de la Prida LM, Huberfeld G, Cohen I, Miles R. Threshold behavior in the initiation of hippocampal population bursts. Neuron. 2006;49:131–142. doi: 10.1016/j.neuron.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Mertens S, Benke D, Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by alpha 5- and delta-subunit-specific immunopurification. J Biol Chem. 1993;268:5965–5973. [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL. Paradoxical reduction of synaptic inhibition by vigabatrin. J Neurophysiol. 2001;86:596–603. doi: 10.1152/jn.2001.86.2.596. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Owens DF, Liu X, Kriegstein AR. Changing properties of GABAA receptor-mediated signaling during early neocortical development. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Marchionni I, Zacchi P, Sieghart W, Cherubini E. Clustering of extrasynaptic GABAA receptors modulates tonic inhibition in cultured hippocampal neurons. J Biol Chem. 2004;279:45833–45843. doi: 10.1074/jbc.M407229200. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J Neurophysiol. 2003;89:1378–1386. doi: 10.1152/jn.00899.2002. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Luddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α 5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Safiulina VF, Fattorini G, Conti F, Cherubini E. GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus. J Neurosci. 2006;26:597–608. doi: 10.1523/JNEUROSCI.4493-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safiulina VF, Kasyanov AM, Sokolova E, Cherubini E, Giniatullin R. ATP contributes to the generation of network-driven giant depolarizing potentials in the neonatal rat hippocampus. J Physiol. 2005;563:981–992. doi: 10.1113/jphysiol.2005.085621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Murgia A, Casula A, Biggio G. Differential subunit dependence of the actions of the general anesthetics alphaxalone and etomidate at gamma-aminobutyric acid type A receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1997;51:484–490. [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Trube G, Mohler H, Malherbe P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- Sipila S, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci. 2005;25:5280–5289. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila S, Huttu K, Voipio J, Kaila K. GABA uptake via GABA transporter-1 modulates GABAergic transmission in the immature hippocampus. J Neurosci. 2004;24:5877–5880. doi: 10.1523/JNEUROSCI.1287-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Chattipakorn SC, McMahon LL. Glycine-gated chloride channels depress synaptic transmission in rat hippocampus. J Neurophysiol. 2006;95:2366–2379. doi: 10.1152/jn.00386.2005. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal α5 subunit-containing gamma-aminobutyric acidA receptors have α5 β3 γ2 pharmacological characteristics. Mol Pharmacol. 1998;54:928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben-Ari Y, Khazipov R. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J Neurophysiol. 2003;90:2964–2972. doi: 10.1152/jn.00172.2003. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. doi: 10.1523/JNEUROSCI.19-23-10372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeyev AY, Cruciani RA, Lange GD, Smallwood VS, Barker JL. Cl− channels are randomly activated by continuous GABA secretion in cultured embryonic rat hippocampal neurons. Neurosci Lett. 1993;155:199–203. doi: 10.1016/0304-3940(93)90707-r. [DOI] [PubMed] [Google Scholar]
- Wall MJ. Furosemide reveals heterogeneous GABAA receptor expression at adult rat Golgi cell to granule cell synapses. Neuropharmacology. 2002;43:737–749. doi: 10.1016/s0028-3908(02)00085-0. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]