Abstract
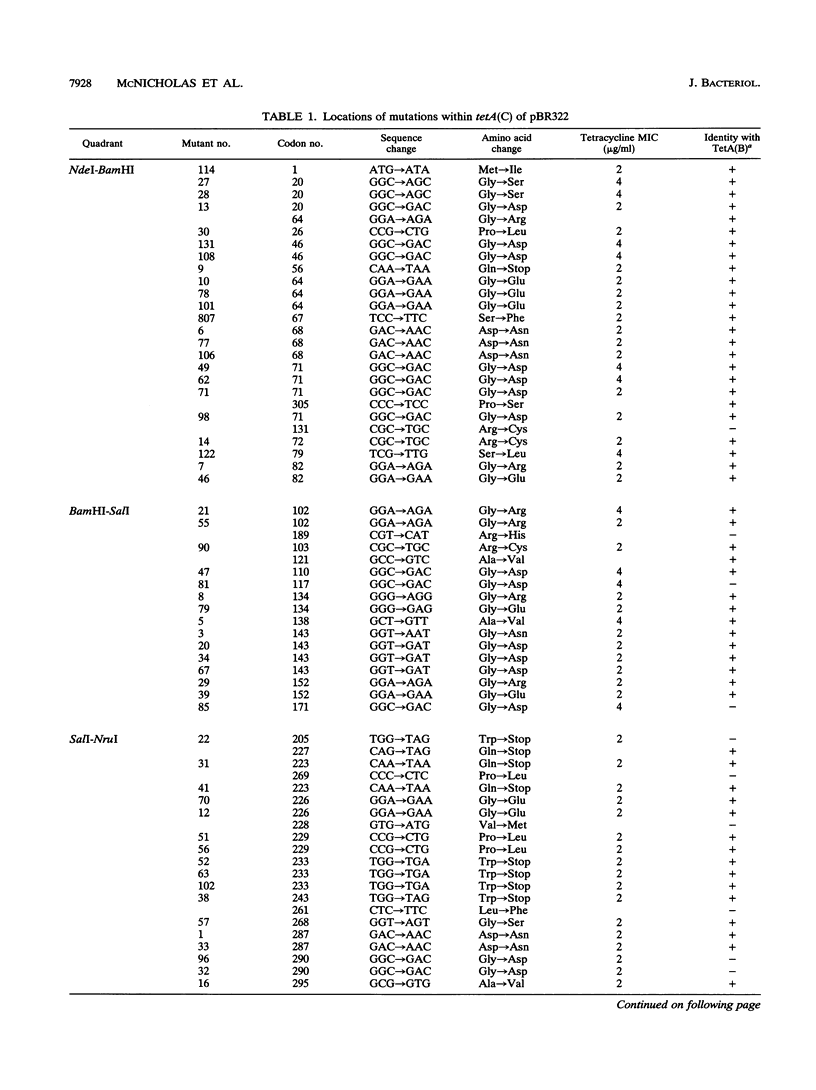
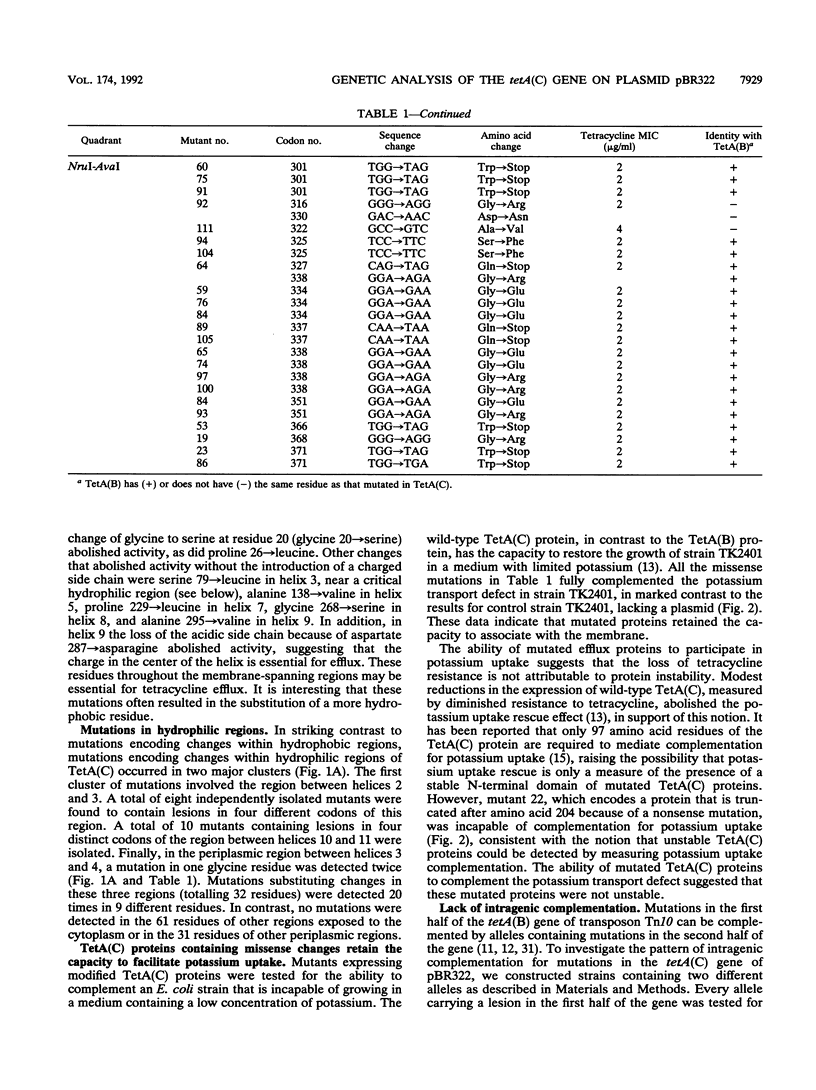
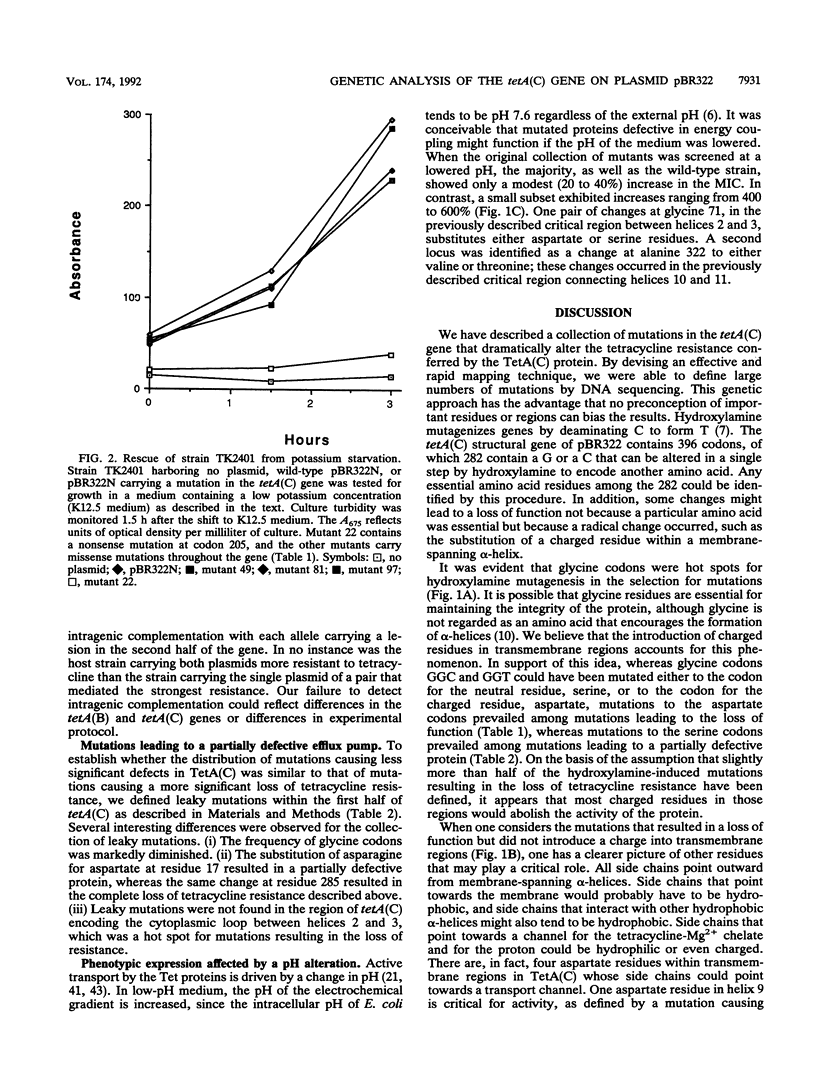
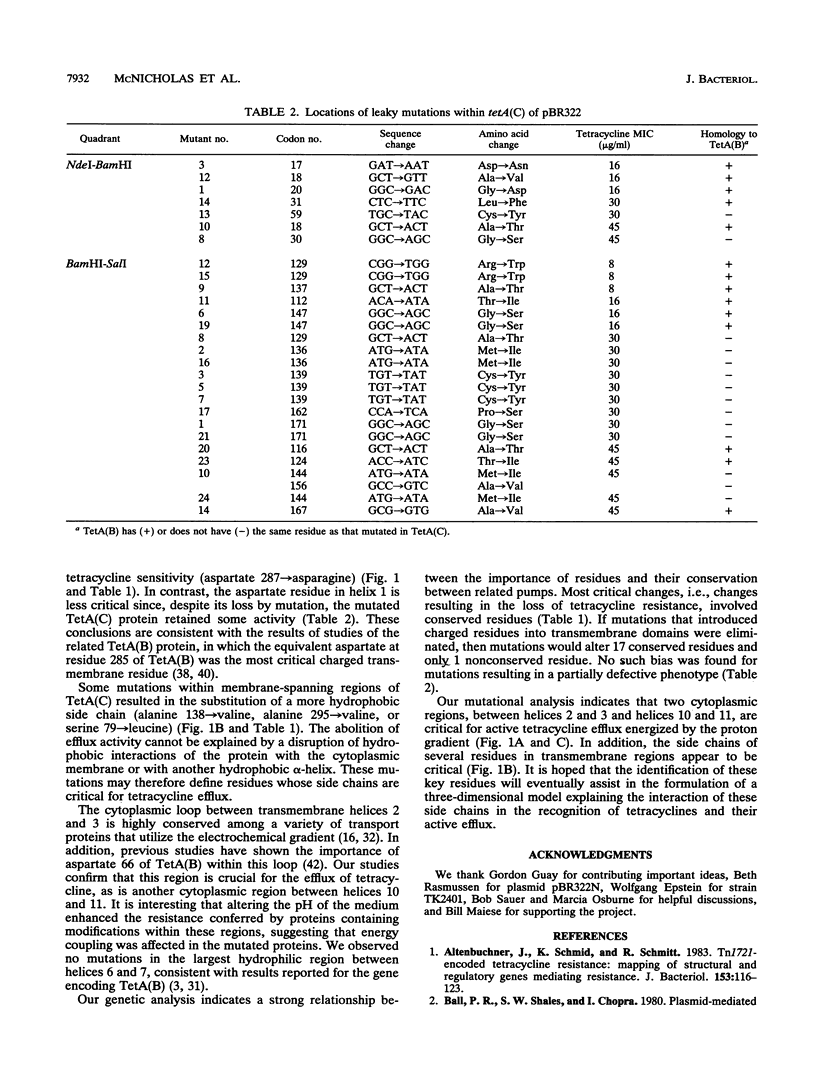
The TetA(C) protein, encoded by the tetA(C) gene of plasmid pBR322, is a member of a family of membrane-bound proteins that mediate energy-dependent efflux of tetracycline from the bacterial cell. The tetA(C) gene was mutagenized with hydroxylamine, and missense mutations causing the loss of tetracycline resistance were identified at 30 distinct codons. Mutations that encoded substitutions within putative membrane-spanning alpha-helical regions were scattered throughout the gene. In contrast, mutations outside the alpha-helical regions were clustered in two cytoplasmic loops, between helices 2 and 3 and helices 10 and 11, suggesting that these regions play a critical role in the recognition of tetracycline and/or energy transduction. All of the missense mutations encoded a protein that retained the ability to rescue an Escherichia coli strain defective in potassium uptake, suggesting that the loss of tetracycline resistance was not due to an unstable TetA(C) protein or to the failure of the protein to be inserted in the membrane. We postulate that the mutations encode residues that are critical for the active efflux of tetracycline, except for mutations that result in the introduction of charged residues within hydrophobic regions of the TetA(C) protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenbuchner J., Schmid K., Schmitt R. Tn1721-encoded tetracycline resistance: mapping of structural and regulatory genes mediating resistance. J Bacteriol. 1983 Jan;153(1):116–123. doi: 10.1128/jb.153.1.116-123.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P. R., Shales S. W., Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochem Biophys Res Commun. 1980 Mar 13;93(1):74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Barany F. Two-codon insertion mutagenesis of plasmid genes by using single-stranded hexameric oligonucleotides. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4202–4206. doi: 10.1073/pnas.82.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. F., Mutzel R., Barbé J., Müller W. A multifunctional gene (tetR) controls Tn10-encoded tetracycline resistance. J Bacteriol. 1982 May;150(2):633–642. doi: 10.1128/jb.150.2.633-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Overlapping divergent promoters control expression of Tn10 tetracycline resistance. Gene. 1983 Aug;23(2):149–156. doi: 10.1016/0378-1119(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budowsky E. I. The mechanism of the mutagenic action of hydroxylamines. Prog Nucleic Acid Res Mol Biol. 1976;16:125–188. doi: 10.1016/s0079-6603(08)60757-6. [DOI] [PubMed] [Google Scholar]
- Chopra I., Hawkey P. M., Hinton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992 Mar;29(3):245–277. doi: 10.1093/jac/29.3.245. [DOI] [PubMed] [Google Scholar]
- Chopra I., Shales S., Ball P. Tetracycline resistance determinants from groups A to D vary in their ability to confer decreased accumulation of tetracycline derivatives by Escherichia coli. J Gen Microbiol. 1982 Apr;128(4):689–692. doi: 10.1099/00221287-128-4-689. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Curiale M. S., Levy S. B. Two complementation groups mediate tetracycline resistance determined by Tn10. J Bacteriol. 1982 Jul;151(1):209–215. doi: 10.1128/jb.151.1.209-215.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiale M. S., McMurry L. M., Levy S. B. Intracistronic complementation of the tetracycline resistance membrane protein of Tn10. J Bacteriol. 1984 Jan;157(1):211–217. doi: 10.1128/jb.157.1.211-217.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch D. C., Salvacion F. F., Epstein W. Tetracycline resistance element of pBR322 mediates potassium transport. J Bacteriol. 1984 Dec;160(3):1188–1190. doi: 10.1128/jb.160.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert B., Beck C. F. Topology of the transposon Tn10-encoded tetracycline resistance protein within the inner membrane of Escherichia coli. J Biol Chem. 1989 Jul 15;264(20):11663–11670. [PubMed] [Google Scholar]
- Griffith J. K., Kogoma T., Corvo D. L., Anderson W. L., Kazim A. L. An N-terminal domain of the tetracycline resistance protein increases susceptibility to aminoglycosides and complements potassium uptake defects in Escherichia coli. J Bacteriol. 1988 Feb;170(2):598–604. doi: 10.1128/jb.170.2.598-604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J. Proton-linked sugar transport systems in bacteria. J Bioenerg Biomembr. 1990 Aug;22(4):525–569. doi: 10.1007/BF00762961. [DOI] [PubMed] [Google Scholar]
- Hillen W., Schollmeier K., Gatz C. Control of expression of the Tn10-encoded tetracycline resistance operon. II. Interaction of RNA polymerase and TET repressor with the tet operon regulatory region. J Mol Biol. 1984 Jan 15;172(2):185–201. doi: 10.1016/s0022-2836(84)80037-6. [DOI] [PubMed] [Google Scholar]
- Hillen W., Schollmeier K. Nucleotide sequence of the Tn10 encoded tetracycline resistance gene. Nucleic Acids Res. 1983 Jan 25;11(2):525–539. doi: 10.1093/nar/11.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Smith H. R., Anderson E. S. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol Gen Genet. 1976 Apr 23;145(1):101–108. doi: 10.1007/BF00331564. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Reznikoff W. S. Organization of structural and regulatory genes that mediate tetracycline resistance in transposon Tn10. J Bacteriol. 1979 Jun;138(3):705–714. doi: 10.1128/jb.138.3.705-714.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M., Yamaguchi A., Sawai T. Energetics of tetracycline efflux system encoded by Tn10 in Escherichia coli. FEBS Lett. 1985 Dec 2;193(2):194–198. doi: 10.1016/0014-5793(85)80149-6. [DOI] [PubMed] [Google Scholar]
- Klock G., Unger B., Gatz C., Hillen W., Altenbuchner J., Schmid K., Schmitt R. Heterologous repressor-operator recognition among four classes of tetracycline resistance determinants. J Bacteriol. 1985 Jan;161(1):326–332. doi: 10.1128/jb.161.1.326-332.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- Marshall B., Morrissey S., Flynn P., Levy S. B. A new tetracycline-resistance determinant, class E, isolated from Enterobacteriaceae. Gene. 1986;50(1-3):111–117. doi: 10.1016/0378-1119(86)90315-x. [DOI] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Nguyen T. T., Postle K., Bertrand K. P. Sequence homology between the tetracycline-resistance determinants of Tn10 and pBR322. Gene. 1983 Nov;25(1):83–92. doi: 10.1016/0378-1119(83)90170-1. [DOI] [PubMed] [Google Scholar]
- Peden K. W. Revised sequence of the tetracycline-resistance gene of pBR322. Gene. 1983 May-Jun;22(2-3):277–280. doi: 10.1016/0378-1119(83)90112-9. [DOI] [PubMed] [Google Scholar]
- Postle K., Nguyen T. T., Bertrand K. P. Nucleotide sequence of the repressor gene of the TN10 tetracycline resistance determinant. Nucleic Acids Res. 1984 Jun 25;12(12):4849–4863. doi: 10.1093/nar/12.12.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. A., Levy S. B. Interdomain hybrid Tet proteins confer tetracycline resistance only when they are derived from closely related members of the tet gene family. J Bacteriol. 1990 May;172(5):2303–2312. doi: 10.1128/jb.172.5.2303-2312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan R. P., Chopra I. Origin of tetracycline efflux proteins: conclusions from nucleotide sequence analysis. Mol Microbiol. 1991 Apr;5(4):895–900. doi: 10.1111/j.1365-2958.1991.tb00763.x. [DOI] [PubMed] [Google Scholar]
- Tovar K., Ernst A., Hillen W. Identification and nucleotide sequence of the class E tet regulatory elements and operator and inducer binding of the encoded purified Tet repressor. Mol Gen Genet. 1988 Dec;215(1):76–80. doi: 10.1007/BF00331306. [DOI] [PubMed] [Google Scholar]
- Unger B., Becker J., Hillen W. Nucleotide sequence of the gene, protein purification and characterization of the pSC101-encoded tetracycline resistance-gene-repressor. Gene. 1984 Nov;31(1-3):103–108. doi: 10.1016/0378-1119(84)90199-9. [DOI] [PubMed] [Google Scholar]
- Unger B., Klock G., Hillen W. Nucleotide sequence of the repressor gene of the RA1 tetracycline resistance determinant: structural and functional comparison with three related Tet repressor genes. Nucleic Acids Res. 1984 Oct 25;12(20):7693–7703. doi: 10.1093/nar/12.20.7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters S. H., Rogowsky P., Grinsted J., Altenbuchner J., Schmitt R. The tetracycline resistance determinants of RP1 and Tn1721: nucleotide sequence analysis. Nucleic Acids Res. 1983 Sep 10;11(17):6089–6105. doi: 10.1093/nar/11.17.6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray L. V., Jr, Jorgensen R. A., Reznikoff W. S. Identification of the tetracycline resistance promoter and repressor in transposon Tn10. J Bacteriol. 1981 Aug;147(2):297–304. doi: 10.1128/jb.147.2.297-304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Adachi K., Akasaka T., Ono N., Sawai T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by a transposon Tn10. Histidine 257 plays an essential role in H+ translocation. J Biol Chem. 1991 Apr 5;266(10):6045–6051. [PubMed] [Google Scholar]
- Yamaguchi A., Adachi K., Sawai T. Orientation of the carboxyl terminus of the transposon Tn10-encoded tetracycline resistance protein in Escherichia coli. FEBS Lett. 1990 Jun 4;265(1-2):17–19. doi: 10.1016/0014-5793(90)80872-g. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Akasaka T., Ono N., Someya Y., Nakatani M., Sawai T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by transposon Tn10. Roles of the aspartyl residues located in the putative transmembrane helices. J Biol Chem. 1992 Apr 15;267(11):7490–7498. [PubMed] [Google Scholar]
- Yamaguchi A., Iwasaki-Ohba Y., Ono N., Kaneko-Ohdera M., Sawai T. Stoichiometry of metal-tetracycline/H+ antiport mediated by transposon Tn10-encoded tetracycline resistance protein in Escherichia coli. FEBS Lett. 1991 May 6;282(2):415–418. doi: 10.1016/0014-5793(91)80527-a. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A., Ono N., Akasaka T., Noumi T., Sawai T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by a transposon, Tn10. The role of the conserved dipeptide, Ser65-Asp66, in tetracycline transport. J Biol Chem. 1990 Sep 15;265(26):15525–15530. [PubMed] [Google Scholar]
- Yamaguchi A., Udagawa T., Sawai T. Transport of divalent cations with tetracycline as mediated by the transposon Tn10-encoded tetracycline resistance protein. J Biol Chem. 1990 Mar 25;265(9):4809–4813. [PubMed] [Google Scholar]


