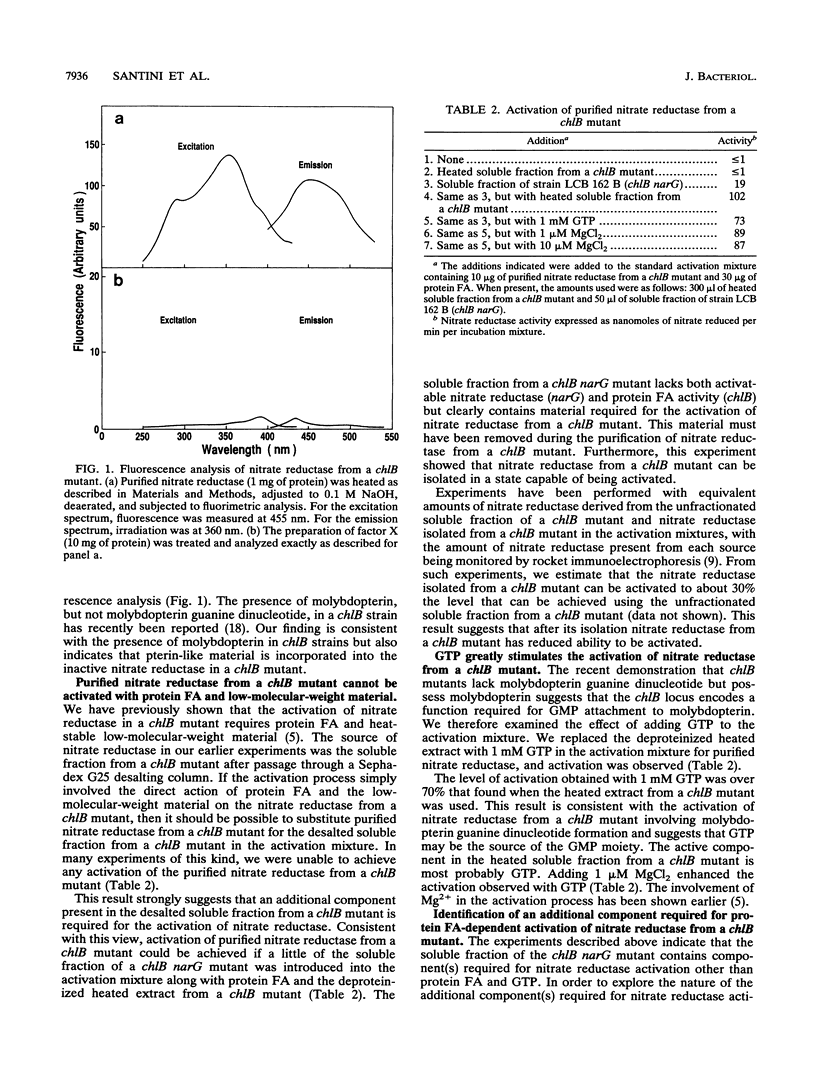
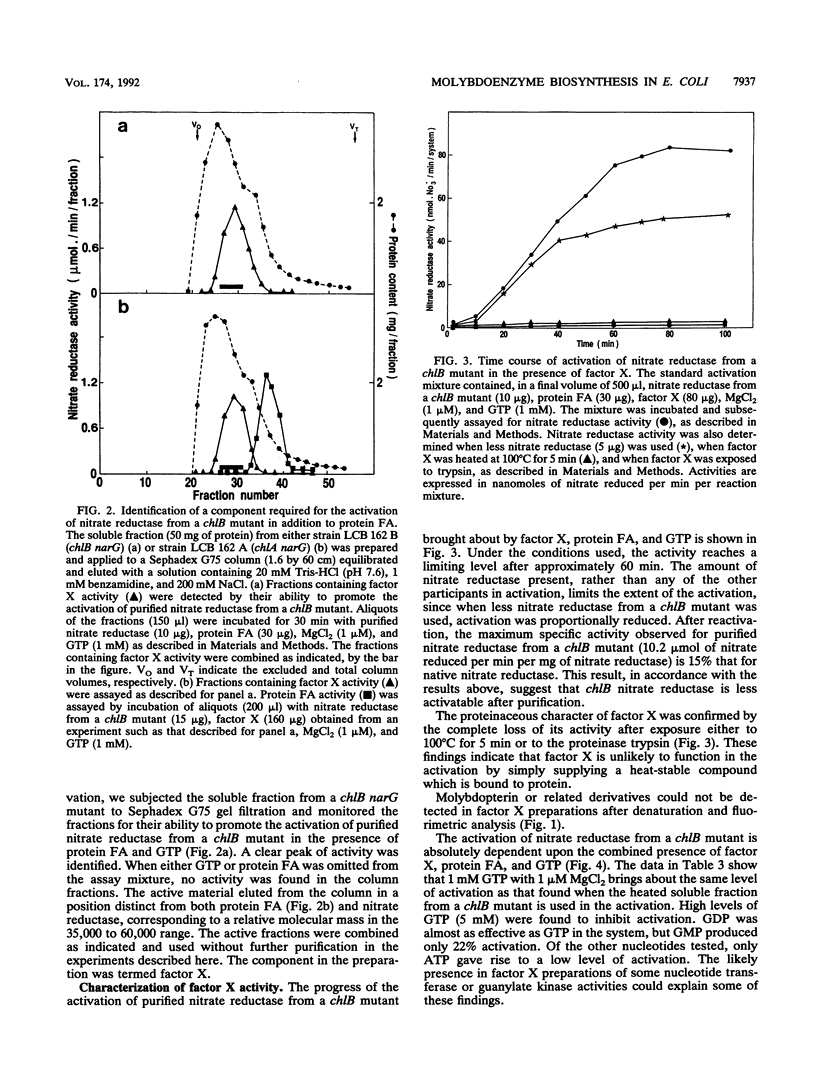
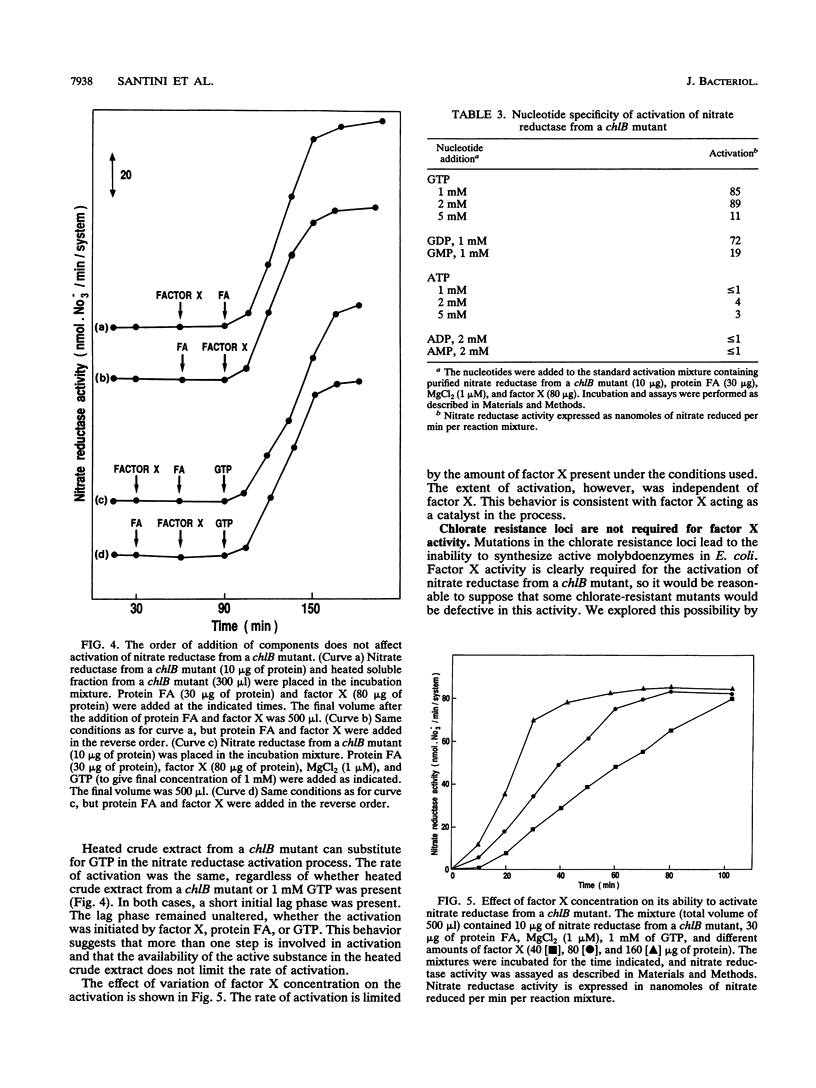
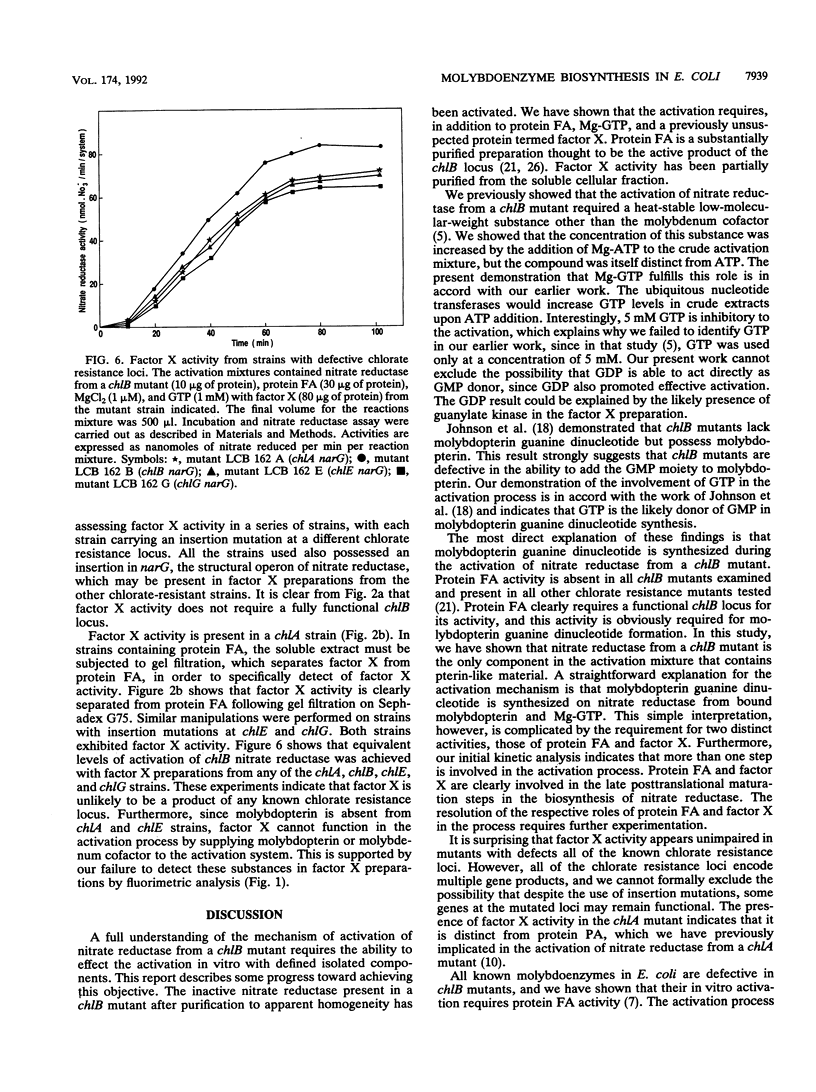
Abstract
All molybdoenzyme activities are absent in chlB mutants because of their inability to synthesize molybdopterin guanine dinucleotide, which together with molybdate constitutes the molybdenum cofactor in Escherichia coli. The chlB mutants are able to synthesize molybdopterin. We have previously shown that the inactive nitrate reductase present in a chlB mutant can be activated in a process requiring protein FA and a heat-stable low-molecular-weight substance. We show here that purified nitrate reductase from the soluble fraction of a chlB mutant can be partially activated in a process that requires protein FA, GTP, and an additional protein termed factor X. It appears that the molybdopterin present in the nitrate reductase of a chlB mutant is converted to molybdopterin guanine dinucleotide during activation. The activation is absolutely dependent upon both protein FA and factor X. Factor X activity is present in chlA, chlB, chlE, and chlG mutants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azoulay E., Puig J., Couchoud-Beaumont P. Etude des mutants chlorate-résistants chez Escherichia coli K 12. I. Reconstitution in vitro de l'activité nitrate-réductase particulaire chez Escherichia coli K 12. Biochim Biophys Acta. 1969 Feb 11;171(2):238–252. doi: 10.1016/0005-2744(69)90157-0. [DOI] [PubMed] [Google Scholar]
- Blasco F., Nunzi F., Pommier J., Brasseur R., Chippaux M., Giordano G. Formation of active heterologous nitrate reductases between nitrate reductases A and Z of Escherichia coli. Mol Microbiol. 1992 Jan;6(2):209–219. doi: 10.1111/j.1365-2958.1992.tb02002.x. [DOI] [PubMed] [Google Scholar]
- Bonnefoy-Orth V., Lepelletier M., Pascal M. C., Chippaux M. Nitrate reductase and cytochrome bnitrate reductase structural genes as parts of the nitrate reductase operon. Mol Gen Genet. 1981;181(4):535–540. doi: 10.1007/BF00428749. [DOI] [PubMed] [Google Scholar]
- Boxer D. H., Low D. C., Pommier J., Giordano G. Involvement of a low-molecular-weight substance in in vitro activation of the molybdoenzyme respiratory nitrate reductase from a chlB mutant of Escherichia coli. J Bacteriol. 1987 Oct;169(10):4678–4685. doi: 10.1128/jb.169.10.4678-4685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G., Boxer D. H., Pommier J. Molybdenum cofactor requirement for in vitro activation of apo-molybdoenzymes of Escherichia coli. Mol Microbiol. 1990 Apr;4(4):645–650. doi: 10.1111/j.1365-2958.1990.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Giordano G., Violet M., Medani C. L., Pommier J. A common pathway for the activation of several molybdoenzymes in Escherichia coli K12. Biochim Biophys Acta. 1984 Apr 10;798(2):216–225. doi: 10.1016/0304-4165(84)90307-6. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet L., Giordano G. Identification and purification of a protein involved in the activation of nitrate reductase in the soluble fraction of a chlA mutant of Escherichia coli K12. Biochim Biophys Acta. 1983 Nov 28;749(1):115–124. doi: 10.1016/0167-4838(83)90158-9. [DOI] [PubMed] [Google Scholar]
- Hinton S. M., Dean D. Biogenesis of molybdenum cofactors. Crit Rev Microbiol. 1990;17(3):169–188. doi: 10.3109/10408419009105724. [DOI] [PubMed] [Google Scholar]
- Iobbi-Nivol C., Santini C. L., Blasco F., Giordano G. Purification and further characterization of the second nitrate reductase of Escherichia coli K12. Eur J Biochem. 1990 Mar 30;188(3):679–687. doi: 10.1111/j.1432-1033.1990.tb15450.x. [DOI] [PubMed] [Google Scholar]
- Johann S., Hinton S. M. Cloning and nucleotide sequence of the chlD locus. J Bacteriol. 1987 May;169(5):1911–1916. doi: 10.1128/jb.169.5.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Bastian N. R., Rajagopalan K. V. Molybdopterin guanine dinucleotide: a modified form of molybdopterin identified in the molybdenum cofactor of dimethyl sulfoxide reductase from Rhodobacter sphaeroides forma specialis denitrificans. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3190–3194. doi: 10.1073/pnas.87.8.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Bastian N. R., Schauer N. L., Ferry J. G., Rajagopalan K. V. Identification of molybdopterin guanine dinucleotide in formate dehydrogenase from Methanobacterium formicicum. FEMS Microbiol Lett. 1991 Jan 15;61(2-3):213–216. doi: 10.1016/0378-1097(91)90554-n. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V., Arison B. H. The pterin component of the molybdenum cofactor. Structural characterization of two fluorescent derivatives. J Biol Chem. 1984 May 10;259(9):5414–5422. [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V. Characterization of the molybdenum cofactor of sulfite oxidase, xanthine, oxidase, and nitrate reductase. Identification of a pteridine as a structural component. J Biol Chem. 1980 Mar 10;255(5):1783–1786. [PubMed] [Google Scholar]
- Johnson J. L., Indermaur L. W., Rajagopalan K. V. Molybdenum cofactor biosynthesis in Escherichia coli. Requirement of the chlB gene product for the formation of molybdopterin guanine dinucleotide. J Biol Chem. 1991 Jul 5;266(19):12140–12145. [PubMed] [Google Scholar]
- Johnson M. E., Rajagopalan K. V. Involvement of chlA, E, M, and N loci in Escherichia coli molybdopterin biosynthesis. J Bacteriol. 1987 Jan;169(1):117–125. doi: 10.1128/jb.169.1.117-125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nohno T., Kasai Y., Saito T. Cloning and sequencing of the Escherichia coli chlEN operon involved in molybdopterin biosynthesis. J Bacteriol. 1988 Sep;170(9):4097–4102. doi: 10.1128/jb.170.9.4097-4102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitterle D. M., Rajagopalan K. V. Two proteins encoded at the chlA locus constitute the converting factor of Escherichia coli chlA1. J Bacteriol. 1989 Jun;171(6):3373–3378. doi: 10.1128/jb.171.6.3373-3378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss J., Kleinhofs A., Klingmüller W. Cloning of seven differently complementing DNA fragments with chl functions from Escherichia coli K12. Mol Gen Genet. 1987 Feb;206(2):352–355. doi: 10.1007/BF00333594. [DOI] [PubMed] [Google Scholar]
- Riviere C., Giordano G., Pommier J., Azoulay E. Membrane reconstitution in chl-r mutants of Escherichia coli K 12. VIII. Purification and properties of the FA factor, the product of the chl B gene. Biochim Biophys Acta. 1975 May 6;389(2):219–235. doi: 10.1016/0005-2736(75)90317-x. [DOI] [PubMed] [Google Scholar]
- Santini C. L., Karibian D., Vasishta A., Boxer D., Giordano G. Escherichia coli molybdoenzymes can be activated by protein FA from several gram-negative bacteria. J Gen Microbiol. 1989 Dec;135(12):3467–3475. doi: 10.1099/00221287-135-12-3467. [DOI] [PubMed] [Google Scholar]
- Saracino L., Violet M., Boxer D. H., Giordano G. Activation in vitro of respiratory nitrate reductase of Escherichia coli K12 grown in the presence of tungstate. Involvement of molybdenum cofactor. Eur J Biochem. 1986 Aug 1;158(3):483–490. doi: 10.1111/j.1432-1033.1986.tb09780.x. [DOI] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


