Abstract
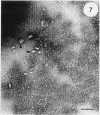
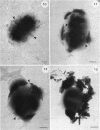
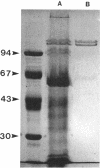
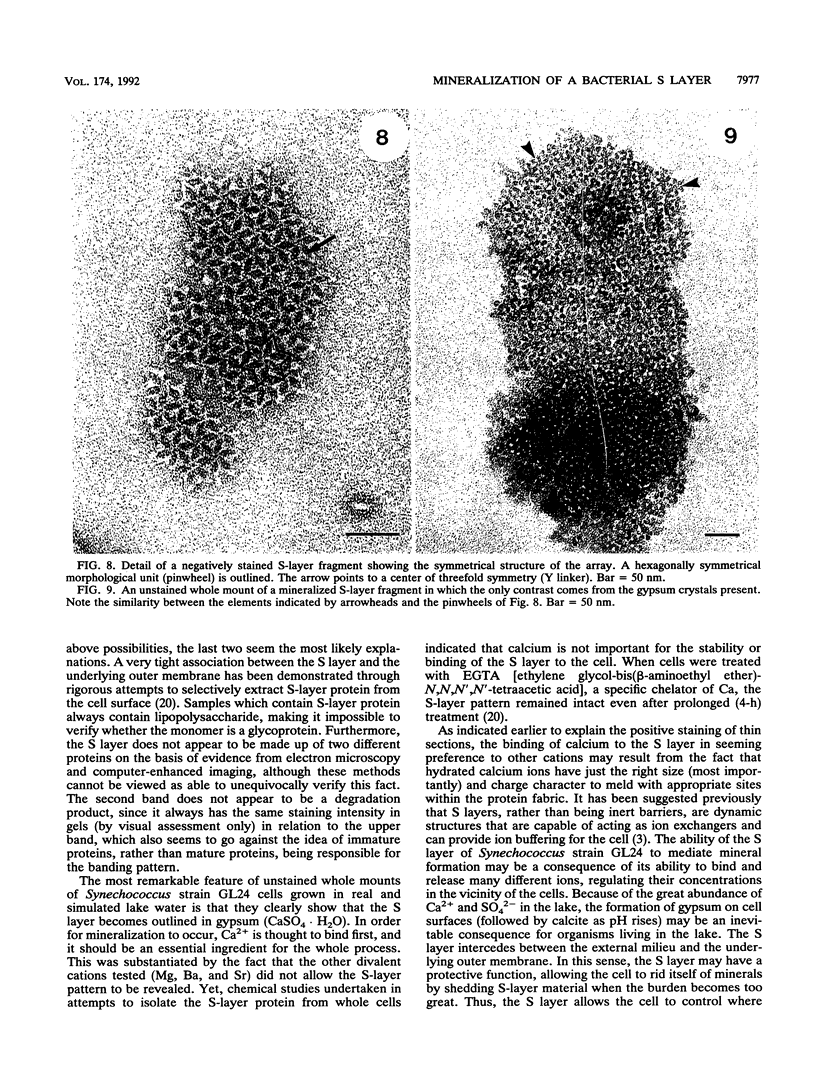
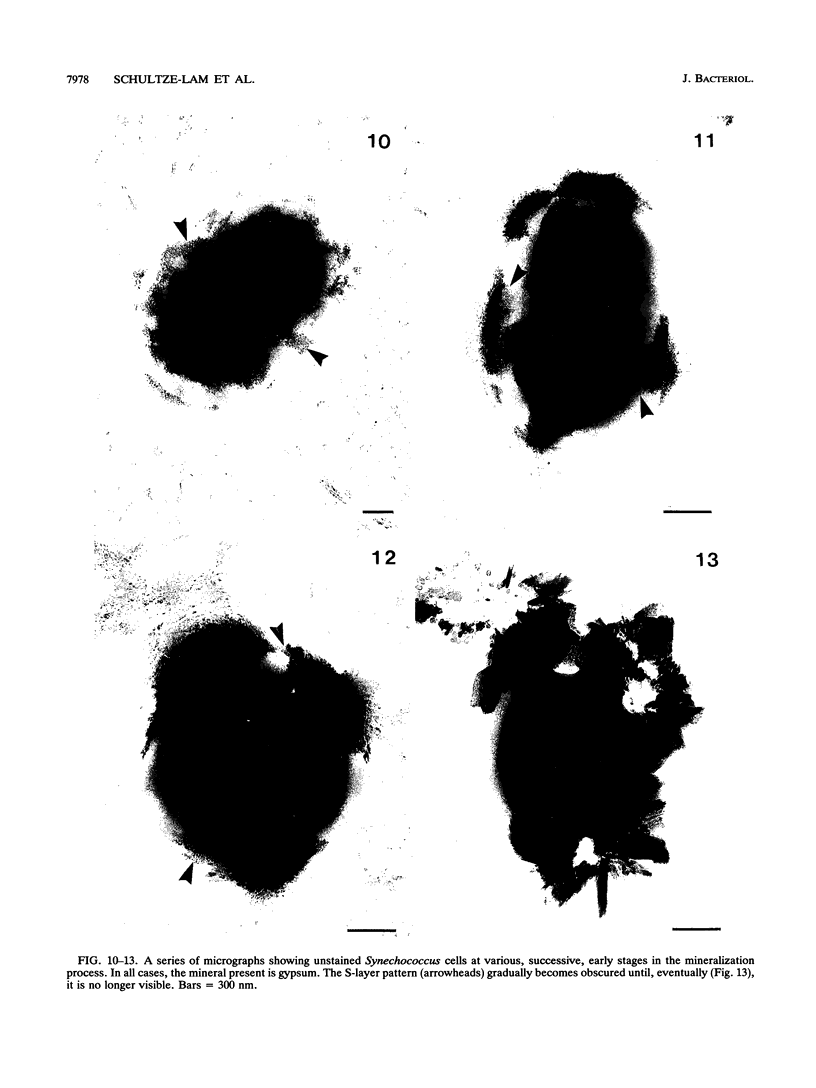
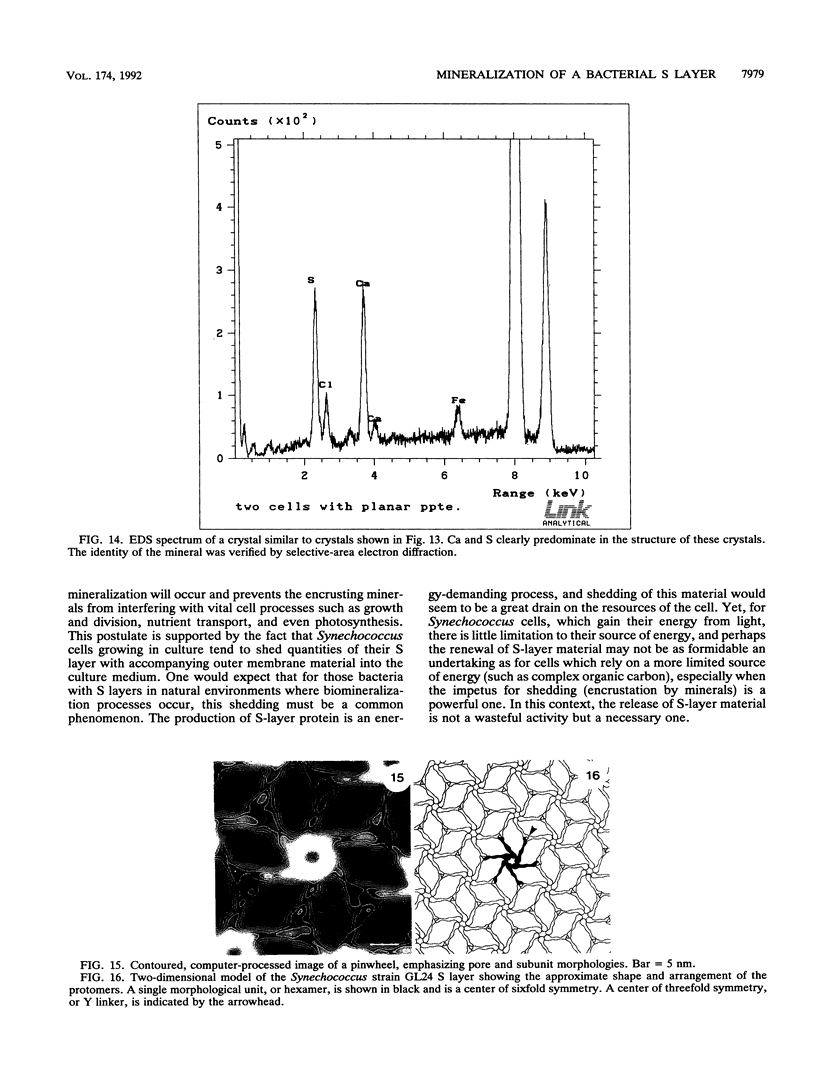
Cyanobacteria belonging to the Synechococcus group are ubiquitous inhabitants of diverse marine and freshwater environments. Through interactions with the soluble constituents of their aqueous habitats, they inevitably affect the chemistry of the waters they inhabit. Synechococcus strain GL24 was isolated from Fayetteville Green Lake, New York, where it has a demonstrated role in the formation of calcitic minerals. In order to understand the detailed interactions which lead to mineral formation by this organism, we have undertaken detailed ultrastructural studies of its cell surface and the initial events in mineral growth using a variety of electron microscopic and computer image enhancement techniques. Synechococcus strain GL24 has a hexagonally symmetrical S layer as its outermost cell surface component. The constituent protein(s) of this structure appears as a double band by sodium dodecyl sulfate-polyacrylamide gel electrophoresis with M(r)s of 104,000 and 109,000. We demonstrate that the S layer acts as a template for fine-grain gypsum and calcite formation by providing discrete, regularly arranged nucleation sites for the critical initial events in the mineralization process. To our knowledge, this is the first time that a bacterial S layer has been shown to have a role in mineral formation in a natural environment, and this report provides conclusive evidence for the specific involvement of bacterial surfaces in natural mineral formation processes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos L. A. Image analysis of macromolecular structures. J Microsc. 1974 Mar;100(2):143–152. doi: 10.1111/j.1365-2818.1974.tb03924.x. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J. Surface arrays on the wall of Sporosarcina ureae. J Bacteriol. 1979 Sep;139(3):1039–1048. doi: 10.1128/jb.139.3.1039-1048.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Evaluation of freeze-substitution and conventional embedding protocols for routine electron microscopic processing of eubacteria. J Bacteriol. 1990 Apr;172(4):2141–2149. doi: 10.1128/jb.172.4.2141-2149.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauz G., Boekema E., van Heel M. Statistical image analysis of electron micrographs of ribosomal subunits. Methods Enzymol. 1988;164:35–49. doi: 10.1016/s0076-6879(88)64033-x. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson B., Vaara T., Lounatmaa K., Gyllenberg H. Three-dimensional structure of the regularly constructed surface layer from Synechocystis sp. strain CLII. J Bacteriol. 1983 Dec;156(3):1338–1343. doi: 10.1128/jb.156.3.1338-1343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lounatmaa K., Vaara T., Osterlund K., Vaara M. Ultrastructure of the cell wall of a Synechocystis strain. Can J Microbiol. 1980 Feb;26(2):204–208. doi: 10.1139/m80-031. [DOI] [PubMed] [Google Scholar]
- Messner P., Pum D., Sára M., Stetter K. O., Sleytr U. B. Ultrastructure of the cell envelope of the archaebacteria Thermoproteus tenax and Thermoproteus neutrophilus. J Bacteriol. 1986 Jun;166(3):1046–1054. doi: 10.1128/jb.166.3.1046-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner P., Sleytr U. B. Crystalline bacterial cell-surface layers. Adv Microb Physiol. 1992;33:213–275. doi: 10.1016/s0065-2911(08)60218-0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. Principles of organization in S layers. J Mol Biol. 1986 Jan 20;187(2):251–253. doi: 10.1016/0022-2836(86)90232-9. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]