Abstract
Bifunctional cross-linking reagents were used to identify cell envelope proteins that interacted with the murein sacculus. This revealed that a number of [3H]leucine-labeled proteins and [3H]palmitate-labeled lipoproteins were reproducibly cross-linked to the sacculus in plasmolyzed cells. The results suggested that most of the cell envelope lipoproteins, and not only the murein lipoprotein, mediate interactions between the murein sacculus and the inner and/or outer membrane of the cell.
Full text
PDF


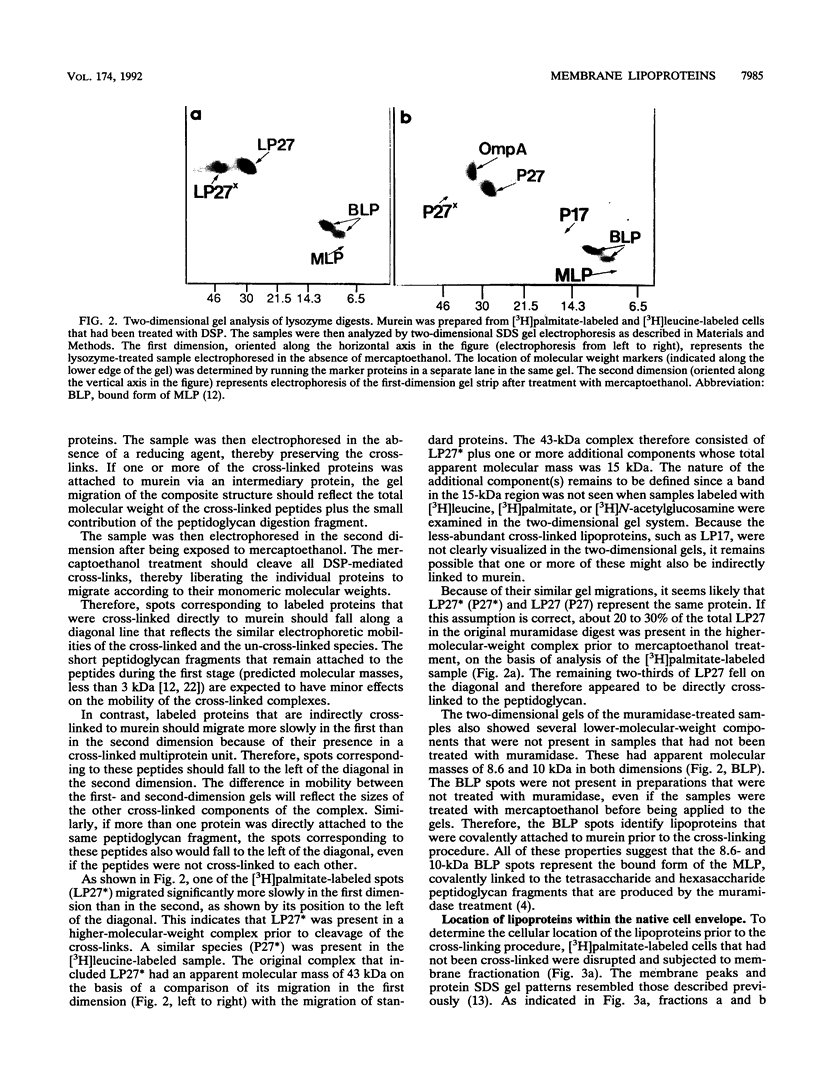
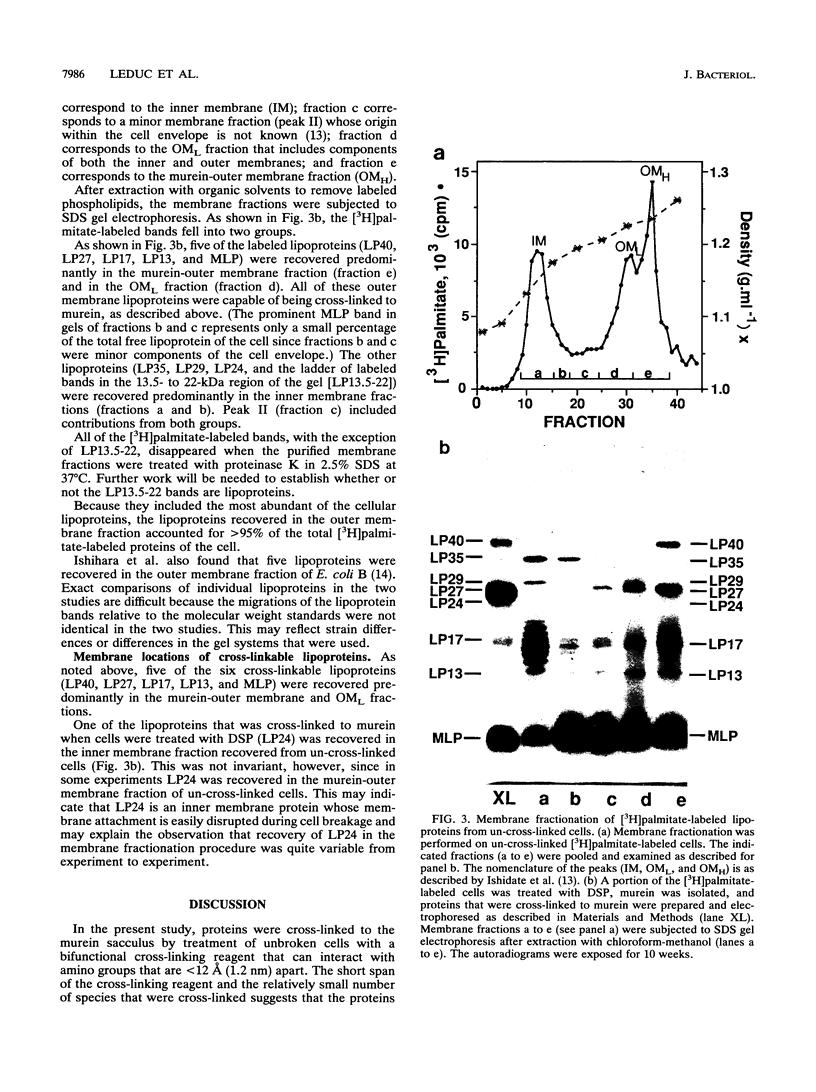


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbas J. A., Díaz J., Rodríguez-Tébar A., Vázquez D. Specific location of penicillin-binding proteins within the cell envelope of Escherichia coli. J Bacteriol. 1986 Jan;165(1):269–275. doi: 10.1128/jb.165.1.269-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Zones of membrane adhesion in the cryofixed envelope of Escherichia coli. J Struct Biol. 1991 Dec;107(3):268–280. doi: 10.1016/1047-8477(91)90052-x. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Cook W. R., MacAlister T. J., Rothfield L. I. Compartmentalization of the periplasmic space at division sites in gram-negative bacteria. J Bacteriol. 1986 Dec;168(3):1430–1438. doi: 10.1128/jb.168.3.1430-1438.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Endermann R., Henning U. Nearest neighbors of major proteins in the outer membrane of Escherichia coli K12. FEBS Lett. 1979 Jan 15;97(2):339–342. doi: 10.1016/0014-5793(79)80117-9. [DOI] [PubMed] [Google Scholar]
- Endermann R., Krämer C., Henning U. Major outer membrane proteins of Escherichia coli K-12: evidence for protein II being a transmembrane protein. FEBS Lett. 1978 Feb 1;86(1):21–24. doi: 10.1016/0014-5793(78)80089-1. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Effects of Dimethylsulfoxide on the Lactose Operon in Escherichia coli. J Bacteriol. 1966 Aug;92(2):353–357. doi: 10.1128/jb.92.2.353-357.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung J., MacAlister T. J., Rothfield L. I. Role of murein lipoprotein in morphogenesis of the bacterial division septum: phenotypic similarity of lkyD and lpo mutants. J Bacteriol. 1978 Mar;133(3):1467–1471. doi: 10.1128/jb.133.3.1467-1471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara S., Hussain M., Mizushima S. Characterization of new membrane lipoproteins and their precursors of Escherichia coli. J Biol Chem. 1981 Mar 25;256(6):3125–3129. [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Ishidate K., Creeger E. S., Zrike J., Deb S., Glauner B., MacAlister T. J., Rothfield L. I. Isolation of differentiated membrane domains from Escherichia coli and Salmonella typhimurium, including a fraction containing attachment sites between the inner and outer membranes and the murein skeleton of the cell envelope. J Biol Chem. 1986 Jan 5;261(1):428–443. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazzaroni J. C., Portalier R. The excC gene of Escherichia coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (PAL). Mol Microbiol. 1992 Mar;6(6):735–742. doi: 10.1111/j.1365-2958.1992.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Leduc M., Frehel C. Characterization of adhesion zones in E. coli cells. FEMS Microbiol Lett. 1990 Jan 15;55(1-2):39–43. doi: 10.1016/0378-1097(90)90164-l. [DOI] [PubMed] [Google Scholar]
- Leduc M., Frehel C., van Heijenoort J. Correlation between degradation and ultrastructure of peptidoglycan during autolysis of Escherichia coli. J Bacteriol. 1985 Feb;161(2):627–635. doi: 10.1128/jb.161.2.627-635.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., Fréhel C., Siegel E., Van Heijenoort J. Multilayered distribution of peptidoglycan in the periplasmic space of Escherichia coli. J Gen Microbiol. 1989 May;135(5):1243–1254. doi: 10.1099/00221287-135-5-1243. [DOI] [PubMed] [Google Scholar]
- Leduc M., Joseleau-Petit D., Rothfield L. I. Interactions of membrane lipoproteins with the murein sacculus of Escherichia coli as shown by chemical crosslinking studies of intact cells. FEMS Microbiol Lett. 1989 Jul 1;51(1):11–14. doi: 10.1016/0378-1097(89)90068-2. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Yashouv-Gan Y., Schwarz U. Peptidoglycan biosynthesis in a thermosensitive division mutant of Escherichia coli. Biochemistry. 1976 May 4;15(9):1781–1790. doi: 10.1021/bi00654a001. [DOI] [PubMed] [Google Scholar]
- Mizuno T. A novel peptidoglycan-associated lipoprotein found in the cell envelope of Pseudomonas aeruginosa and Escherichia coli. J Biochem. 1979 Oct;86(4):991–1000. doi: 10.1093/oxfordjournals.jbchem.a132631. [DOI] [PubMed] [Google Scholar]
- Palva E. T. Protein interactions in the outer membrane of Escherichia coli. Eur J Biochem. 1979 Feb 1;93(3):495–503. doi: 10.1111/j.1432-1033.1979.tb12848.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tébar A., Barbas J. A., Vázquez D. Location of some proteins involved in peptidoglycan synthesis and cell division in the inner and outer membranes of Escherichia coli. J Bacteriol. 1985 Jan;161(1):243–248. doi: 10.1128/jb.161.1.243-248.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Yasuda S., Nishimura A., Yamada M., Hirota Y. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol Gen Genet. 1978 Nov 16;167(1):1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- Weigand R. A., Vinci K. D., Rothfield L. I. Morphogenesis of the bacterial division septum: a new class of septation-defective mutants. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1882–1886. doi: 10.1073/pnas.73.6.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]