Abstract
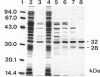
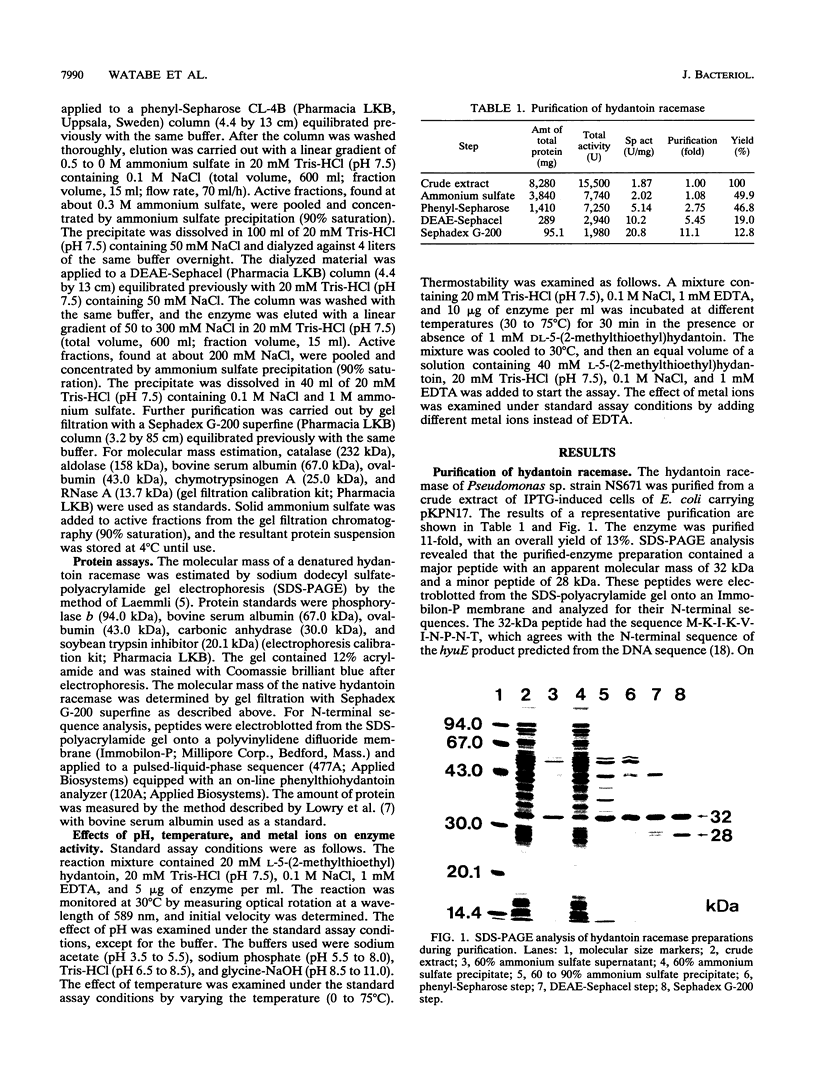
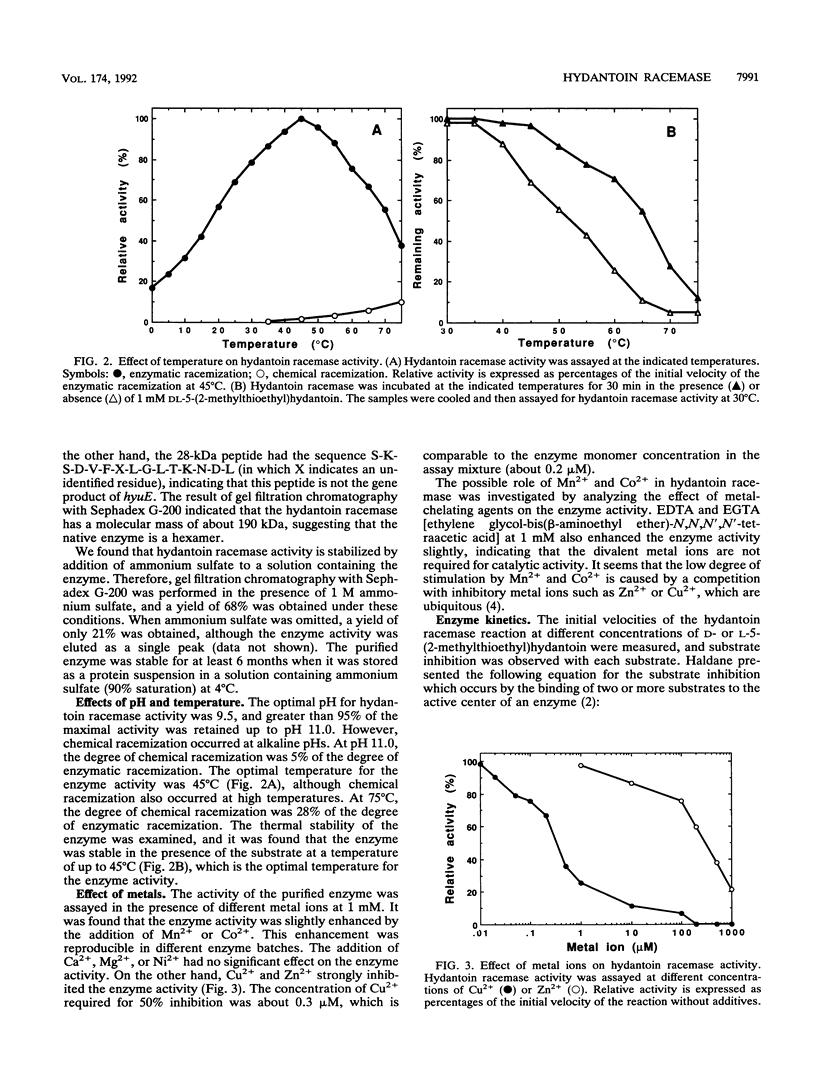
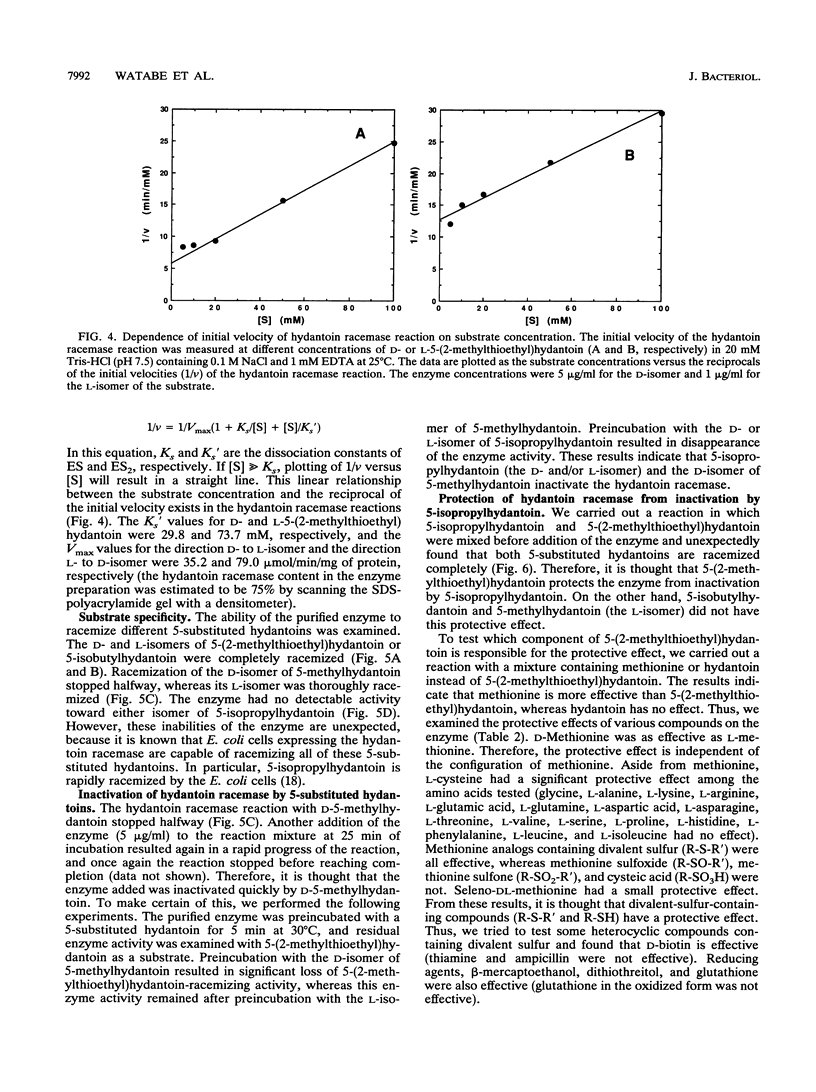
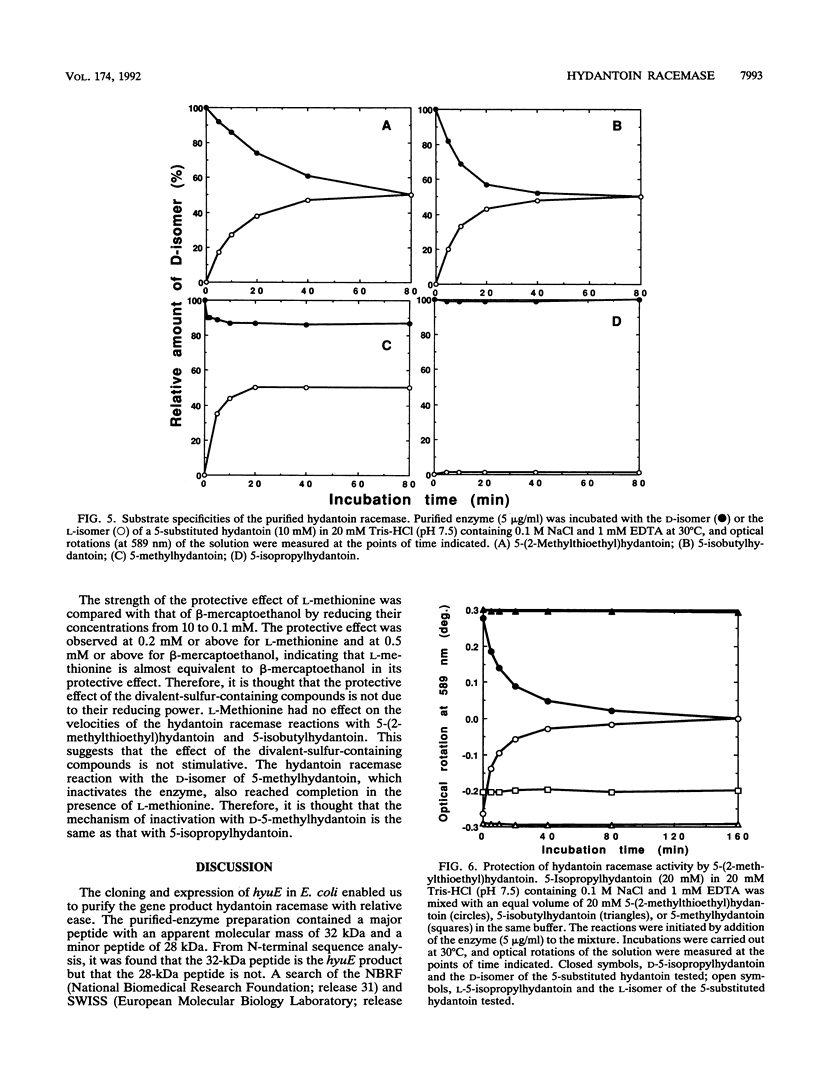
The hydantoin racemase gene of Pseudomonas sp. strain NS671 had been cloned and expressed in Escherichia coli. Hydantoin racemase was purified from the cell extract of the E. coli strain by phenyl-Sepharose, DEAE-Sephacel, and Sephadex G-200 chromatographies. The purified enzyme had an apparent molecular mass of 32 kDa as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. By gel filtration, a molecular mass of about 190 kDa was found, suggesting that the native enzyme is a hexamer. The optimal conditions for hydantoin racemase activity were pH 9.5 and a temperature of 45 degrees C. The enzyme activity was slightly stimulated by the addition of not only Mn2+ or Co2+ but also metal-chelating agents, indicating that the enzyme is not a metalloenzyme. On the other hand, Cu2+ and Zn2+ strongly inhibited the enzyme activity. Kinetic studies showed substrate inhibition, and the Vmax values for D- and L-5-(2-methylthioethyl)hydantoin were 35.2 and 79.0 mumol/min/mg of protein, respectively. The purified enzyme did not racemize 5-isopropylhydantoin, whereas the cells of E. coli expressing the enzyme are capable of racemizing it. After incubation of the purified enzyme with 5-isopropylhydantoin, the enzyme no longer showed 5-(2-methylthioethyl)hydantoin-racemizing activity. However, in the presence of 5-(2-methylthioethyl)hydantoin, the purified enzyme racemized 5-isopropylhydantoin completely, suggesting that 5-(2-methylthioethyl)hydantoin protects the enzyme from inactivation by 5-isopropylhydratoin. Thus, we examined the protective effect of various compounds and found that divalent-sulfur-containing compounds (R-S-R' and R-SH) have this protective effect.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook W. J., Koszalka G. W., Hall W. W., Narayana S. V., Ealick S. E. Crystallization and preliminary X-ray investigation of uridine phosphorylase from Escherichia coli. J Biol Chem. 1987 Feb 25;262(6):2852–2853. [PubMed] [Google Scholar]
- Helmkamp G. M., Jr, Brock D. J., Bloch K. Beta-hydroxydecanoly thioester dehydrase. Specificity of substrates and acetylenic inhibitors. J Biol Chem. 1968 Jun 25;243(12):3229–3231. [PubMed] [Google Scholar]
- Holmquist B. Elimination of adventitious metals. Methods Enzymol. 1988;158:6–12. doi: 10.1016/0076-6879(88)58042-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leer J. C., Hammer-Jespersen K., Schwartz M. Uridine phosphorylase from Escherichia coli. Physical and chemical characterization. Eur J Biochem. 1977 May 2;75(1):217–224. doi: 10.1111/j.1432-1033.1977.tb11520.x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- STARK G. R., SMYTH D. G. The use of cyanate for the determination of NH2-terminal residues in proteins. J Biol Chem. 1963 Jan;238:214–226. [PubMed] [Google Scholar]
- Sheth K., Alexander J. K. Purification and properties of beta-1,4-oligoglucan:orthophosphate glucosyltransferase from Clostridium thermocellum. J Biol Chem. 1969 Jan 25;244(2):457–464. [PubMed] [Google Scholar]
- Soffer R. L. Enzymatic modification of proteins. II. Purification and properties of the arginyl transfer ribonucleic acid-protein transferase from rabbit liver cytoplasm. J Biol Chem. 1970 Feb 25;245(4):731–737. [PubMed] [Google Scholar]
- Stegink L. D., Coon M. J. Stereospecificity and other properties of highly purified beta-hydroxy-beta-methylglutaryl coenzyme A cleavage enzyme from bovine liver. J Biol Chem. 1968 Oct 25;243(20):5272–5279. [PubMed] [Google Scholar]
- Uyeda K., Rabinowitz J. C. Pyruvate-ferredoxin oxidoreductase. 3. Purification and properties of the enzyme. J Biol Chem. 1971 May 25;246(10):3111–3119. [PubMed] [Google Scholar]
- Walsh C. T. Suicide substrates, mechanism-based enzyme inactivators: recent developments. Annu Rev Biochem. 1984;53:493–535. doi: 10.1146/annurev.bi.53.070184.002425. [DOI] [PubMed] [Google Scholar]
- Watabe K., Ishikawa T., Mukohara Y., Nakamura H. Cloning and sequencing of the genes involved in the conversion of 5-substituted hydantoins to the corresponding L-amino acids from the native plasmid of Pseudomonas sp. strain NS671. J Bacteriol. 1992 Feb;174(3):962–969. doi: 10.1128/jb.174.3.962-969.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe K., Ishikawa T., Mukohara Y., Nakamura H. Identification and sequencing of a gene encoding a hydantoin racemase from the native plasmid of Pseudomonas sp. strain NS671. J Bacteriol. 1992 Jun;174(11):3461–3466. doi: 10.1128/jb.174.11.3461-3466.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]