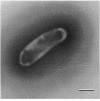
Abstract
In the course of a study on the bacterial degradation of plant cell wall polysaccharides, we observed that growing cells of motile cellulolytic bacteria accumulated, without attachment, near cellulose fibers present in the cultures. Because it seemed likely that the accumulation was due to chemotactic behavior, we investigated the chemotactic responses of one of the above-mentioned bacteria (Cellulomonas gelida ATCC 488). We studied primarily the responses toward cellobiose, which is the major product of cellulose hydrolysis by microorganisms, and toward hemicellulose hydrolysis products. We found that cellobiose, cellotriose, D-glucose, xylobiose, and D-xylose, as well as other sugars that are hemicellulose components, served as chemoattractants for C. gelida, as determined by a modification of Adler's capillary assay. Competition and inducibility experiments indicated that C. gelida possesses at least two types of separately regulated cellobiose chemoreceptors (Cb1 and cellobiose, cellotriose, xylobiose, and D-glucose, and it is constitutively synthesized. The presence in C. gelida of a constitutive response toward cellobiose and of at least two distinct cellobiose chemoreceptors has implications for the survival of this cellulolytic bacterium in nature. A possible mechanism for cellobiose-mediated bacterial chemotaxis toward cellulose is proposed. We suggest that, in natural environments, motile cellulolytic bacteria migrate toward plant materials that contain cellulose and hemicellulose by swimming up cellobiose concentration gradients and/or concentration gradients of other sugars (e.g., xylobiose, D-xylose, and D-glucose) formed by enzymatic hydrolysis of plant cell wall polysaccharides.
Full text
PDF

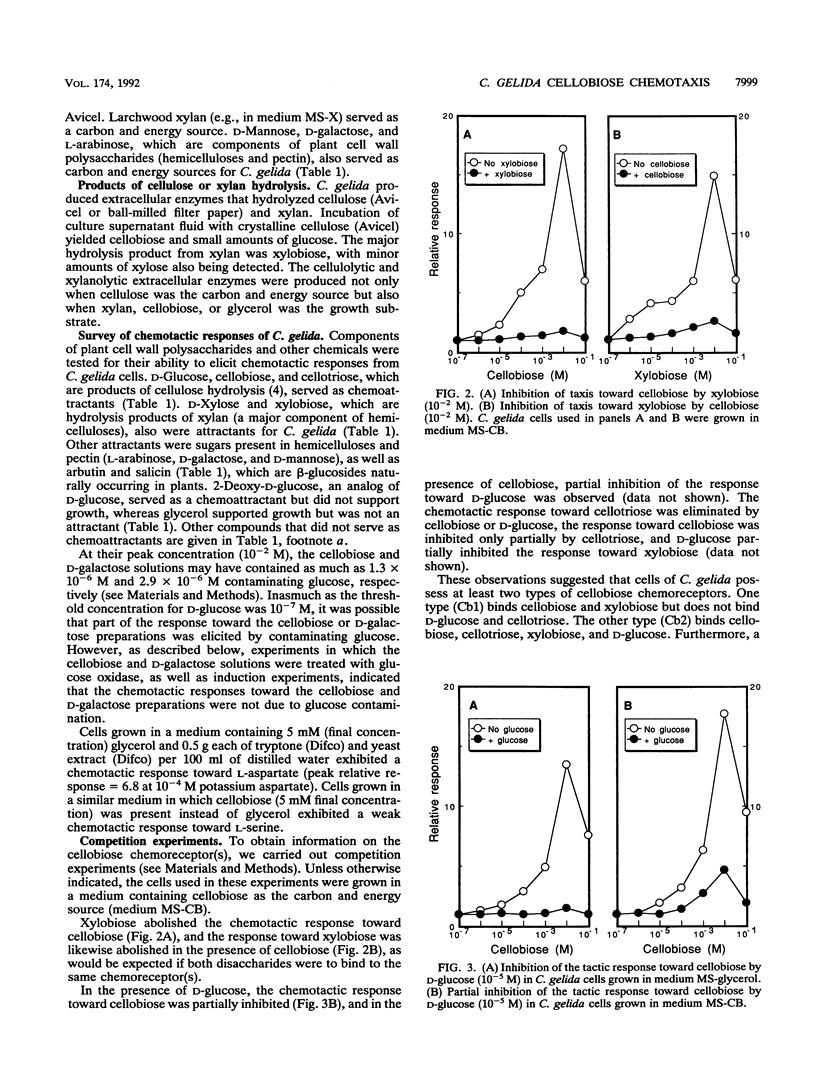



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Hazelbauer G. L., Dahl M. M. Chemotaxis toward sugars in Escherichia coli. J Bacteriol. 1973 Sep;115(3):824–847. doi: 10.1128/jb.115.3.824-847.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavedon K., Leschine S. B., Canale-Parola E. Cellulase system of a free-living, mesophilic clostridium (strain C7). J Bacteriol. 1990 Aug;172(8):4222–4230. doi: 10.1128/jb.172.8.4222-4230.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavedon K., Leschine S. B., Canale-Parola E. Characterization of the extracellular cellulase from a mesophilic clostridium (strain C7). J Bacteriol. 1990 Aug;172(8):4231–4237. doi: 10.1128/jb.172.8.4231-4237.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Chemotaxis in Spirochaeta aurantia. J Bacteriol. 1977 Apr;130(1):485–494. doi: 10.1128/jb.130.1.485-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungate R. E. Studies on Cellulose Fermentation: I. The Culture and Physiology of an Anaerobic Cellulose-digesting Bacterium. J Bacteriol. 1944 Nov;48(5):499–513. doi: 10.1128/jb.48.5.499-513.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschine S. B., Canale-Parola E. Mesophilic cellulolytic clostridia from freshwater environments. Appl Environ Microbiol. 1983 Sep;46(3):728–737. doi: 10.1128/aem.46.3.728-737.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschine S. B., Holwell K., Canale-Parola E. Nitrogen fixation by anaerobic cellulolytic bacteria. Science. 1988 Nov 25;242(4882):1157–1159. doi: 10.1126/science.242.4882.1157. [DOI] [PubMed] [Google Scholar]
- Mesibov R., Adler J. Chemotaxis toward amino acids in Escherichia coli. J Bacteriol. 1972 Oct;112(1):315–326. doi: 10.1128/jb.112.1.315-326.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Villani D. P., Rosendahl M. S. Chemotaxis towards sugars by Bacillus subtilis. J Gen Microbiol. 1979 Nov;115(1):167–172. doi: 10.1099/00221287-115-1-167. [DOI] [PubMed] [Google Scholar]
- Saddler J. N., Khan A. W., Martin S. M. Regulation of cellulase synthesis in Acetivibrio cellulolyticus. Microbios. 1980;28(112):97–106. [PubMed] [Google Scholar]
- Stanton T. B., Canale-Parola E. Treponema bryantii sp. nov., a rumen spirochete that interacts with cellulolytic bacteria. Arch Microbiol. 1980 Sep;127(2):145–156. doi: 10.1007/BF00428018. [DOI] [PubMed] [Google Scholar]
- Terracciano J. S., Canale-Parola E. Enhancement of chemotaxis in Spirochaeta aurantia grown under conditions of nutrient limitation. J Bacteriol. 1984 Jul;159(1):173–178. doi: 10.1128/jb.159.1.173-178.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis R. M., Chasalow S., Koshland D. E., Jr The role of methylation in chemotaxis. An explanation of outstanding anomalies. J Biol Chem. 1990 Apr 25;265(12):6817–6826. [PubMed] [Google Scholar]
- Weis R. M., Koshland D. E., Jr Chemotaxis in Escherichia coli proceeds efficiently from different initial tumble frequencies. J Bacteriol. 1990 Feb;172(2):1099–1105. doi: 10.1128/jb.172.2.1099-1105.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]