Abstract
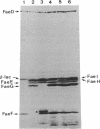
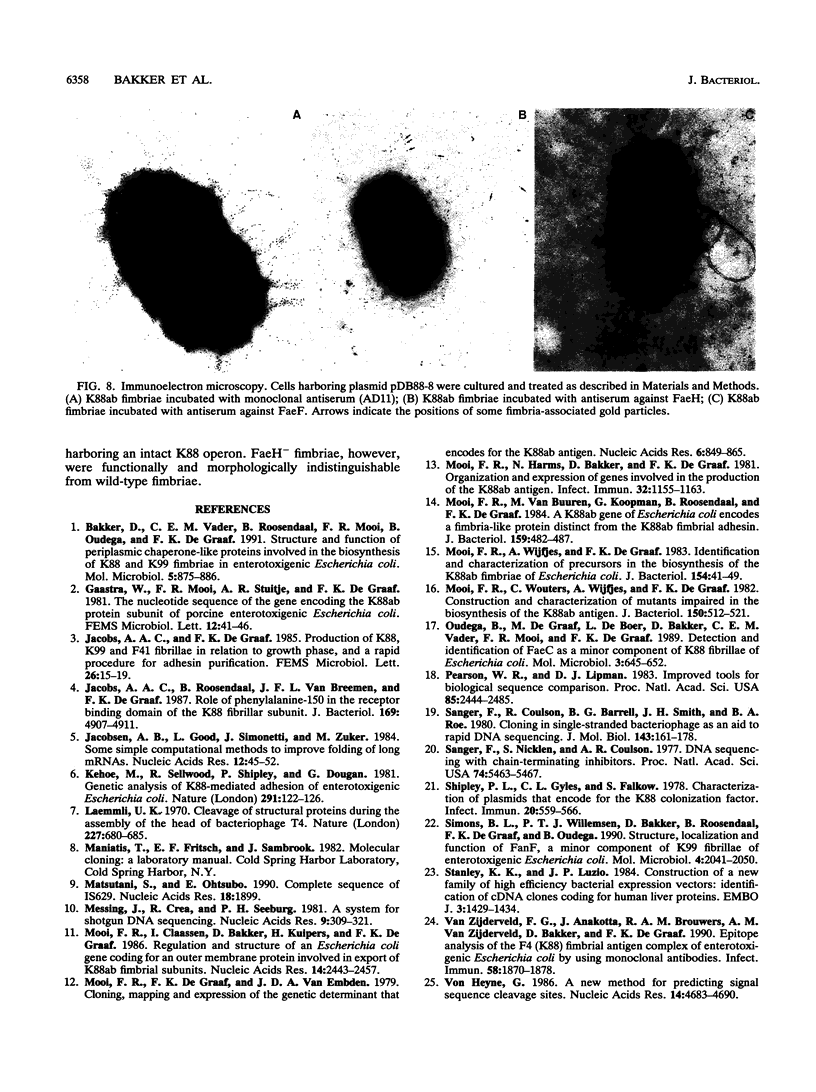
The nucleotide sequences of the genes faeF, faeH, faeI, and faeJ encoding K88 minor fimbrial subunits were determined. Analysis of the primary structure of the gene products revealed that all four proteins are synthesized with an amino-terminal signal sequence. The molecular masses of the mature FaeF, FaeH, FaeI, and FaeJ proteins were calculated to be 15,161, 25,461, 24,804, and 25,093 Da, respectively. FaeH, FaeI, and FaeJ showed significant homology with FaeG, the major fimbrial subunit of K88 fimbriae. Mutations in the respective genes were constructed. Analysis of the mutants showed that the minor fimbrial subunits FaeF and FaeH play an essential role in the biogenesis but not in the adhesive properties of the K88 fimbriae. Mutations in faeI or faeJ had no significant effect on K88 production or adhesive capacity. Specific antisera against FaeF and FaeH were raised by immunization with hybrid Cro-LacZ-FaeF and Cro-LacZ-FaeH proteins. Immunoblotting and immunoelectron microscopy revealed that FaeF and FaeH are located in or along the K88 fimbrial structure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker D., Vader C. E., Roosendaal B., Mooi F. R., Oudega B., de Graaf F. K. Structure and function of periplasmic chaperone-like proteins involved in the biosynthesis of K88 and K99 fimbriae in enterotoxigenic Escherichia coli. Mol Microbiol. 1991 Apr;5(4):875–886. doi: 10.1111/j.1365-2958.1991.tb00761.x. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., Roosendaal B., van Breemen J. F., de Graaf F. K. Role of phenylalanine 150 in the receptor-binding domain of the K88 fibrillar subunit. J Bacteriol. 1987 Nov;169(11):4907–4911. doi: 10.1128/jb.169.11.4907-4911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. B., Good L., Simonetti J., Zuker M. Some simple computational methods to improve the folding of large RNAs. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):45–52. doi: 10.1093/nar/12.1part1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M., Sellwood R., Shipley P., Dougan G. Genetic analysis of K88-mediated adhesion of enterotoxigenic Escherichia coli. Nature. 1981 May 14;291(5811):122–126. doi: 10.1038/291122a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsutani S., Ohtsubo E. Complete sequence of IS629. Nucleic Acids Res. 1990 Apr 11;18(7):1899–1899. doi: 10.1093/nar/18.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Claassen I., Bakker D., Kuipers H., de Graaf F. K. Regulation and structure of an Escherichia coli gene coding for an outer membrane protein involved in export of K88ab fimbrial subunits. Nucleic Acids Res. 1986 Mar 25;14(6):2443–2457. doi: 10.1093/nar/14.6.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Harms N., Bakker D., de Graaf F. K. Organization and expression of genes involved in the production of the K88ab antigen. Infect Immun. 1981 Jun;32(3):1155–1163. doi: 10.1128/iai.32.3.1155-1163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Wijfjes A., de Graaf F. K. Identification and characterization of precursors in the biosynthesis of the K88ab fimbria of Escherichia coli. J Bacteriol. 1983 Apr;154(1):41–49. doi: 10.1128/jb.154.1.41-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., Wouters C., Wijfjes A., de Graaf F. K. Construction and characterization of mutants impaired in the biosynthesis of the K88ab antigen. J Bacteriol. 1982 May;150(2):512–521. doi: 10.1128/jb.150.2.512-521.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., de Graaf F. K., van Embden J. D. Cloning, mapping and expression of the genetic determinant that encodes for the K88ab antigen. Nucleic Acids Res. 1979 Mar;6(3):849–865. doi: 10.1093/nar/6.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi F. R., van Buuren M., Koopman G., Roosendaal B., de Graaf F. K. K88ab gene of Escherichia coli encodes a fimbria-like protein distinct from the K88ab fimbrial adhesin. J Bacteriol. 1984 Aug;159(2):482–487. doi: 10.1128/jb.159.2.482-487.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., de Graaf M., de Boer L., Bakker D., Vader C. E., Mooi F. R., de Graaf F. K. Detection and identification of FaeC as a minor component of K88 fibrillae of Escherichia coli. Mol Microbiol. 1989 May;3(5):645–652. doi: 10.1111/j.1365-2958.1989.tb00212.x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley P. L., Gyles C. L., Falkow S. Characterization of plasmids that encode for the K88 colonization antigen. Infect Immun. 1978 May;20(2):559–566. doi: 10.1128/iai.20.2.559-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons B. L., Willemsen P. T., Bakker D., Roosendaal B., De Graaf F. K., Oudega B. Structure, localization and function of FanF, a minor component of K99 fibrillae of enterotoxigenic Escherichia coli. Mol Microbiol. 1990 Dec;4(12):2041–2050. doi: 10.1111/j.1365-2958.1990.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijderveld F. G., Anakotta J., Brouwers R. A., van Zijderveld A. M., Bakker D., de Graaf F. K. Epitope analysis of the F4 (K88) fimbrial antigen complex of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect Immun. 1990 Jun;58(6):1870–1878. doi: 10.1128/iai.58.6.1870-1878.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]