Abstract
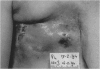
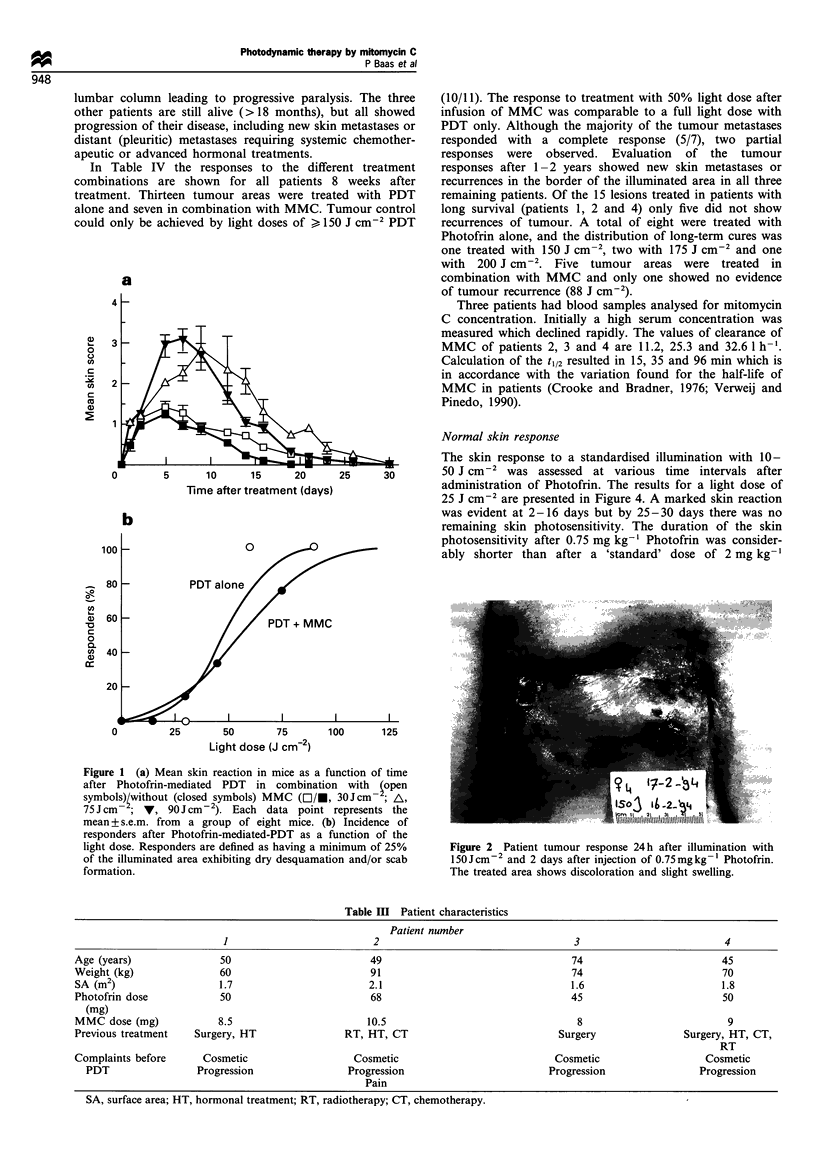
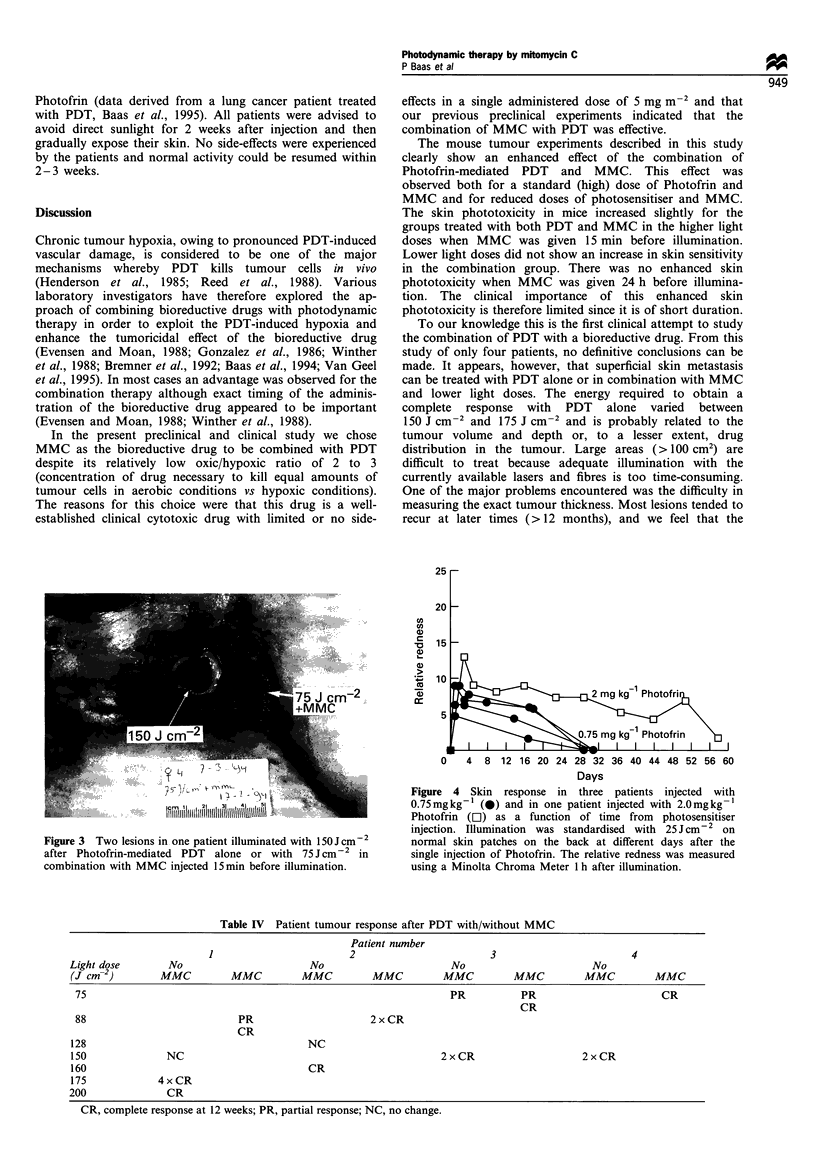
Photodynamic therapy (PDT) using Photofrin was used in combination with a hypoxic toxin (mitomycin C, MMC) to treat four patients with recurrent skin metastasis of a mammary carcinoma. In preclinical experiments an additive effect was found for the combination of MMC and PDT for treating subcutaneous RIF1 tumours in mice. When interstitial PDT was combined with a low dose of MMC (administered 15 min before illumination), the Photofrin dose or light dose could be reduced by a factor of 2 in order to obtain equivalent cure rate or growth delay. In the clinical pilot study, a low dose of Photofrin (0.75 mg kg-1) was used for PDT alone (superficial illumination) or combined with low-dose MMC (5 mg m-2). Different tumour areas were illuminated with or without a preceding infusion of MMC. Both tumour response and skin photosensitivity were scored. After 8-12 weeks of treatment, tumour cure could be achieved by administering light doses > or = 150 J cm-2 for PDT alone and similar effects were obtained when light doses of 75-87.5 J cm-2 were given after infusion with MMC. In all cases necrotic tissue of both tumour and surrounding skin was observed, which lasted for a mean of 5 months (range 2-20 months). Skin phototoxicity, tested by using a standardised illumination of skin patches on the back, lasted maximally 3 weeks. Three main conclusions could be drawn from these studies: (1) The enhanced effects of the combination of PDT and MMC observed in mouse tumours can be extrapolated to patients with mammary skin metastasis. (2) The combination of PDT and hypoxic toxins facilitates treatment by permitting lower doses of photosensitiser to be used (thereby reducing skin phototoxicity) or lower light doses (thereby reducing illumination times and allowing the possibility to treat larger tumour areas). (3) Restoration of skin after PDT in previously treated tumour areas (chemotherapy, radiation therapy and surgery) is very low.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas P., Oppelaar H., Stavenuiter M., van Zandwijk N., Stewart F. A. Interaction of the bioreductive drug SR 4233 and photodynamic therapy using photofrin in a mouse tumor model. Int J Radiat Oncol Biol Phys. 1993 Oct 20;27(3):665–670. doi: 10.1016/0360-3016(93)90394-b. [DOI] [PubMed] [Google Scholar]
- Baas P., Oppelaar H., van der Valk M. A., van Zandwijk N., Stewart F. A. Partial protection of photodynamic-induced skin reactions in mice by N-acetylcysteine: a preclinical study. Photochem Photobiol. 1994 Apr;59(4):448–454. doi: 10.1111/j.1751-1097.1994.tb05063.x. [DOI] [PubMed] [Google Scholar]
- Baas P., van Mansom I., van Tinteren H., Stewart F. A., van Zandwijk N. Effect of N-acetylcysteïne on Photofrin-induced skin photosensitivity in patients. Lasers Surg Med. 1995;16(4):359–367. doi: 10.1002/lsm.1900160407. [DOI] [PubMed] [Google Scholar]
- Bandieramonte G., Marchesini R., Melloni E., Andreoli C., di Pietro S., Spinelli P., Fava G., Zunino F., Emanuelli H. Laser phototherapy following HpD administration in superficial neoplastic lesions. Tumori. 1984 Aug 31;70(4):327–334. doi: 10.1177/030089168407000406. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Kol R., Riklis E., Marko R., Rosenthal I. Effect of light fluence rate on mammalian cells photosensitization by chloroaluminium phthalocyanine tetrasulphonate. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Mar;51(3):467–476. doi: 10.1080/09553008714550951. [DOI] [PubMed] [Google Scholar]
- Bremner J. C., Adams G. E., Pearson J. K., Sansom J. M., Stratford I. J., Bedwell J., Bown S. G., MacRobert A. J., Phillips D. Increasing the effect of photodynamic therapy on the RIF-1 murine sarcoma, using the bioreductive drugs RSU1069 and RB6145. Br J Cancer. 1992 Dec;66(6):1070–1076. doi: 10.1038/bjc.1992.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. C., Stratford I. J., Bowler J., Adams G. E. Bioreductive drugs and the selective induction of tumour hypoxia. Br J Cancer. 1990 May;61(5):717–721. doi: 10.1038/bjc.1990.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairnduff F., Stringer M. R., Hudson E. J., Ash D. V., Brown S. B. Superficial photodynamic therapy with topical 5-aminolaevulinic acid for superficial primary and secondary skin cancer. Br J Cancer. 1994 Mar;69(3):605–608. doi: 10.1038/bjc.1994.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. H., Straight R. C., Smith J. A., Jr Effects of photodynamic therapy in combination with intravesical drugs in a murine bladder tumor model. J Urol. 1992 Mar;147(3):743–746. doi: 10.1016/s0022-5347(17)37370-6. [DOI] [PubMed] [Google Scholar]
- Cowled P. A., Forbes I. J. Modification by vasoactive drugs of tumour destruction by photodynamic therapy with haematoporphyrin derivative. Br J Cancer. 1989 Jun;59(6):904–909. doi: 10.1038/bjc.1989.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooke S. T., Bradner W. T. Mitomycin C: a review. Cancer Treat Rev. 1976 Sep;3(3):121–139. doi: 10.1016/s0305-7372(76)80019-9. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Cooper M. T., Mang T. S. Cutaneous phototoxic occurrences in patients receiving Photofrin. Lasers Surg Med. 1990;10(5):485–488. doi: 10.1002/lsm.1900100514. [DOI] [PubMed] [Google Scholar]
- Fingar V. H., Mang T. S., Henderson B. W. Modification of photodynamic therapy-induced hypoxia by fluosol-DA (20%) and carbogen breathing in mice. Cancer Res. 1988 Jun 15;48(12):3350–3354. [PubMed] [Google Scholar]
- Gomer C. J., Ferrario A., Hayashi N., Rucker N., Szirth B. C., Murphree A. L. Molecular, cellular, and tissue responses following photodynamic therapy. Lasers Surg Med. 1988;8(5):450–463. doi: 10.1002/lsm.1900080503. [DOI] [PubMed] [Google Scholar]
- Gonzalez S., Arnfield M. R., Meeker B. E., Tulip J., Lakey W. H., Chapman J. D., McPhee M. S. Treatment of Dunning R3327-AT rat prostate tumors with photodynamic therapy in combination with misonidazole. Cancer Res. 1986 Jun;46(6):2858–2862. [PubMed] [Google Scholar]
- Henderson B. W., Fingar V. H. Oxygen limitation of direct tumor cell kill during photodynamic treatment of a murine tumor model. Photochem Photobiol. 1989 Mar;49(3):299–304. doi: 10.1111/j.1751-1097.1989.tb04110.x. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Waldow S. M., Mang T. S., Potter W. R., Malone P. B., Dougherty T. J. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985 Feb;45(2):572–576. [PubMed] [Google Scholar]
- Khan S. A., Dougherty T. J., Mang T. S. An evaluation of photodynamic therapy in the management of cutaneous metastases of breast cancer. Eur J Cancer. 1993;29A(12):1686–1690. doi: 10.1016/0959-8049(93)90105-o. [DOI] [PubMed] [Google Scholar]
- Koren H., Alth G., Schenk G. M., Jindra R. H. Photodynamic therapy--an alternative pathway in the treatment of recurrent breast cancer. Int J Radiat Oncol Biol Phys. 1994 Jan 15;28(2):463–466. doi: 10.1016/0360-3016(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Ma L. W., Moan J., Berg K., Peng Q., Steen H. B. Potentiation of photodynamic therapy by mitomycin C in cultured human colon adenocarcinoma cells. Radiat Res. 1993 Apr;134(1):22–28. [PubMed] [Google Scholar]
- Reed M. W., Miller F. N., Wieman T. J., Tseng M. T., Pietsch C. G. The effect of photodynamic therapy on the microcirculation. J Surg Res. 1988 Nov;45(5):452–459. doi: 10.1016/0022-4804(88)90195-3. [DOI] [PubMed] [Google Scholar]
- Schuh M., Nseyo U. O., Potter W. R., Dao T. L., Dougherty T. J. Photodynamic therapy for palliation of locally recurrent breast carcinoma. J Clin Oncol. 1987 Nov;5(11):1766–1770. doi: 10.1200/JCO.1987.5.11.1766. [DOI] [PubMed] [Google Scholar]
- Sperduto P. W., DeLaney T. F., Thomas G., Smith P., Dachowski L. J., Russo A., Bonner R., Glatstein E. Photodynamic therapy for chest wall recurrence in breast cancer. Int J Radiat Oncol Biol Phys. 1991 Jul;21(2):441–446. doi: 10.1016/0360-3016(91)90793-4. [DOI] [PubMed] [Google Scholar]
- Star W. M., Marijnissen H. P., van den Berg-Blok A. E., Versteeg J. A., Franken K. A., Reinhold H. S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986 May;46(5):2532–2540. [PubMed] [Google Scholar]
- Verweij J., Pinedo H. M. Mitomycin C: mechanism of action, usefulness and limitations. Anticancer Drugs. 1990 Oct;1(1):5–13. [PubMed] [Google Scholar]
- Wilson B. D., Mang T. S., Stoll H., Jones C., Cooper M., Dougherty T. J. Photodynamic therapy for the treatment of basal cell carcinoma. Arch Dermatol. 1992 Dec;128(12):1597–1601. [PubMed] [Google Scholar]
- Winther J., Overgaard J., Ehlers N. The effect of photodynamic therapy alone and in combination with misonidazole or X-rays for management of a retinoblastoma-like tumour. Photochem Photobiol. 1988 Mar;47(3):419–423. doi: 10.1111/j.1751-1097.1988.tb02746.x. [DOI] [PubMed] [Google Scholar]
- van Geel I. P., Oppelaar H., Oussoren Y. G., Schuitmaker J. J., Stewart F. A. Mechanisms for optimising photodynamic therapy: second-generation photosensitisers in combination with mitomycin C. Br J Cancer. 1995 Aug;72(2):344–350. doi: 10.1038/bjc.1995.336. [DOI] [PMC free article] [PubMed] [Google Scholar]