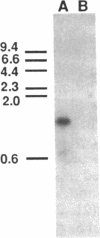
Abstract
Treponema phagedenis is a host-associated spirochete with multiple polypeptides making up its periplasmic flagella (PFs). Each PF has a 39-kDa polypeptide making up the sheath (class A PF polypeptide) and two to four antigenically similar 33- to 34-kDa polypeptide species making up the core (class B PF polypeptides). A genetic analysis of the PF-deficient mutants T-40 and T-55 has shown that the PFs are involved in motility. To better understand the synthesis and assembly of these complex organelles and to compare the PF genes with those of other spirochetes, we cloned and characterized the T. phagedenis flaB2 gene, which encodes one class B polypeptide. The flaB2 gene consists of an open reading frame of 858 nucleotides capable of encoding a protein of 31.5 kDa. The predicted amino acid sequence of the FlaB2 polypeptide was 92% identical to that of T. pallidum FlaB2, with a 76% identity at the nucleotide level. These results confirm previous immunological and N-terminal-sequence analyses which suggested that the PF genes are well conserved in the spirochetes. Primer extension analysis of T. phagedenis flaB2 indicated that the start site of transcription was 127 nucleotides upstream from the ATG initiation codon. Preceding the start site is a DNA sequence similar to the sigma 28 consensus promoter sequence commonly found associated with motility genes. Northern (RNA) blots probed with a segment of flaB2 DNA revealed a 1,000-nucleotide monocistronic transcript in the wild type and in PF-deficient mutants T-40 and T-55. DNA sequencing of most of the flaB2 gene of the mutants revealed no differences from the wild-type gene. Because the mutants fail to synthesize detectable class B PF polypeptides yet synthesize extensive amounts of flaB2 mRNA, PF synthesis in T. phagedenis is likely to involve regulation at the translational level.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnosti D. N., Chamberlin M. J. Secondary sigma factor controls transcription of flagellar and chemotaxis genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):830–834. doi: 10.1073/pnas.86.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. How spirochetes may swim. J Theor Biol. 1976 Feb;56(2):269–273. doi: 10.1016/s0022-5193(76)80074-4. [DOI] [PubMed] [Google Scholar]
- Brahamsha B., Greenberg E. P. Biochemical and cytological analysis of the complex periplasmic flagella from Spirochaeta aurantia. J Bacteriol. 1988 Sep;170(9):4023–4032. doi: 10.1128/jb.170.9.4023-4032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley D. B., Charon N. W. Axial filament involvement in the motility of Leptospira interrogans. J Bacteriol. 1979 Mar;137(3):1406–1412. doi: 10.1128/jb.137.3.1406-1412.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Champion C. I., Miller J. N., Lovett M. A., Blanco D. R. Cloning, sequencing, and expression of two class B endoflagellar genes of Treponema pallidum subsp. pallidum encoding the 34.5- and 31.0-kilodalton proteins. Infect Immun. 1990 Jun;58(6):1697–1704. doi: 10.1128/iai.58.6.1697-1704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Goldstein S. F., Block S. M., Curci K., Ruby J. D., Kreiling J. A., Limberger R. J. Morphology and dynamics of protruding spirochete periplasmic flagella. J Bacteriol. 1992 Feb;174(3):832–840. doi: 10.1128/jb.174.3.832-840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Goldstein S. F., Curci K., Limberger R. J. The bent-end morphology of Treponema phagedenis is associated with short, left-handed, periplasmic flagella. J Bacteriol. 1991 Aug;173(15):4820–4826. doi: 10.1128/jb.173.15.4820-4826.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Chang J. Y., Shaper J. H., Glazer A. N. Amino acid sequence of flagellin of Bacillus subtilis 168. III. Tryptic peptides, N-bromosuccinimide peptides, and the complete amino acid sequence. J Biol Chem. 1976 Feb 10;251(3):705–711. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. F., Charon N. W. Motility of the spirochete Leptospira. Cell Motil Cytoskeleton. 1988;9(2):101–110. doi: 10.1002/cm.970090202. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. DNA sequence analysis suggests that expression of flagellar and chemotaxis genes in Escherichia coli and Salmonella typhimurium is controlled by an alternative sigma factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6422–6424. doi: 10.1073/pnas.84.18.6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma M., Fujita H., Yamaguchi S., Iino T. Excretion of unassembled flagellin by Salmonella typhimurium mutants deficient in hook-associated proteins. J Bacteriol. 1984 Sep;159(3):1056–1059. doi: 10.1128/jb.159.3.1056-1059.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs R. D., Hanke J. H., Guzman-Verduzco L. M., Newport G., Agabian N., Norgard M. V., Lukehart S. A., Radolf J. D. Molecular cloning and DNA sequence analysis of the 37-kilodalton endoflagellar sheath protein gene of Treponema pallidum. Infect Immun. 1989 Nov;57(11):3403–3411. doi: 10.1128/iai.57.11.3403-3411.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joys T. M., Rankis V. The primary structure of the phase-1 flagellar protein of Salmonella typhimurium. I. The tryptic peptides. J Biol Chem. 1972 Aug 25;247(16):5180–5193. [PubMed] [Google Scholar]
- Kimsey R. B., Spielman A. Motility of Lyme disease spirochetes in fluids as viscous as the extracellular matrix. J Infect Dis. 1990 Nov;162(5):1205–1208. doi: 10.1093/infdis/162.5.1205. [DOI] [PubMed] [Google Scholar]
- Kutsukake K., Ohya Y., Iino T. Transcriptional analysis of the flagellar regulon of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):741–747. doi: 10.1128/jb.172.2.741-747.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima G., Asaka J., Fujiwara T., Fujiwara T., Node K., Kondo E. Nucleotide sequence of the hag gene encoding flagellin of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1479–1483. doi: 10.1128/jb.168.3.1479-1483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger R. J., Charon N. W. Antiserum to the 33,000-dalton periplasmic-flagellum protein of "Treponema phagedenis" reacts with other treponemes and Spirochaeta aurantia. J Bacteriol. 1986 Nov;168(2):1030–1032. doi: 10.1128/jb.168.2.1030-1032.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberger R. J., Charon N. W. Treponema phagedenis has at least two proteins residing together on its periplasmic flagella. J Bacteriol. 1986 Apr;166(1):105–112. doi: 10.1128/jb.166.1.105-112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Martin J. H., Savage D. C. Cloning, nucleotide sequence, and taxonomic implications of the flagellin gene of Roseburia cecicola. J Bacteriol. 1988 Jun;170(6):2612–2617. doi: 10.1128/jb.170.6.2612-2617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Fieldsteel A. H. Genetics of Treponema: relationship between Treponema pallidum and five cultivable treponemes. J Bacteriol. 1978 Jan;133(1):101–107. doi: 10.1128/jb.133.1.101-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison M., Rood J. I., Faine S., Adler B. Molecular analysis of a Leptospira borgpetersenii gene encoding an endoflagellar subunit protein. J Gen Microbiol. 1991 Jul;137(7):1529–1536. doi: 10.1099/00221287-137-7-1529. [DOI] [PubMed] [Google Scholar]
- Nell E. E., Hardy P. H., Jr Counterimmunoelectrophoresis of Reiter Treponeme Axial filaments as a diagnostic test for syphilis. J Clin Microbiol. 1978 Aug;8(2):148–152. doi: 10.1128/jcm.8.2.148-152.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Charon N. W., Cook R. G., Fuentes M. D., Limberger R. J. Antigenic relatedness and N-terminal sequence homology define two classes of periplasmic flagellar proteins of Treponema pallidum subsp. pallidum and Treponema phagedenis. J Bacteriol. 1988 Sep;170(9):4072–4082. doi: 10.1128/jb.170.9.4072-4082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallesen L., Hindersson P. Cloning and sequencing of a Treponema pallidum gene encoding a 31.3-kilodalton endoflagellar subunit (FlaB2). Infect Immun. 1989 Jul;57(7):2166–2172. doi: 10.1128/iai.57.7.2166-2172.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parales J., Jr, Greenberg E. P. N-terminal amino acid sequences and amino acid compositions of the Spirochaeta aurantia flagellar filament polypeptides. J Bacteriol. 1991 Feb;173(3):1357–1359. doi: 10.1128/jb.173.3.1357-1359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Involvement of periplasmic fibrils in motility of spirochetes. J Bacteriol. 1980 Jan;141(1):359–364. doi: 10.1128/jb.141.1.359-364.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Dewhirst F. E., Weisburg W. G., Tordoff L. A., Fraser G. J., Hespell R. B., Stanton T. B., Zablen L., Mandelco L., Woese C. R. Phylogenetic analysis of the spirochetes. J Bacteriol. 1991 Oct;173(19):6101–6109. doi: 10.1128/jb.173.19.6101-6109.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen N. S., Petersen C. S., Vejtorp M., Axelsen N. H. Serodiagnosis of syphilis by an enzyme-linked immunosorbent assay for IgG antibodies against the Reiter treponeme flagellum. Scand J Immunol. 1982 Apr;15(4):341–348. doi: 10.1111/j.1365-3083.1982.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Blanco D. R., Miller J. N., Lovett M. A. Antigenic interrelationship between endoflagella of Treponema phagedenis biotype Reiter and Treponema pallidum (Nichols): molecular characterization of endoflagellar proteins. Infect Immun. 1986 Dec;54(3):626–634. doi: 10.1128/iai.54.3.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sadziene A., Thomas D. D., Bundoc V. G., Holt S. C., Barbour A. G. A flagella-less mutant of Borrelia burgdorferi. Structural, molecular, and in vitro functional characterization. J Clin Invest. 1991 Jul;88(1):82–92. doi: 10.1172/JCI115308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. I., Emerson S. U., Shaper J. H., Bernard P. D., Glazer A. N. Classification of Bacillus subtilis flagellins. J Bacteriol. 1977 Apr;130(1):200–204. doi: 10.1128/jb.130.1.200-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strugnell R., Cockayne A., Penn C. W. Molecular and antigenic analysis of treponemes. Crit Rev Microbiol. 1990;17(4):231–250. doi: 10.3109/10408419009105727. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Navab M., Haake D. A., Fogelman A. M., Miller J. N., Lovett M. A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc Natl Acad Sci U S A. 1988 May;85(10):3608–3612. doi: 10.1073/pnas.85.10.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Moter S. E., Simon M. M., Ebnet K., Heiberger A., Kramer M. D. The Borrelia burgdorferi flagellum-associated 41-kilodalton antigen (flagellin): molecular cloning, expression, and amplification of the gene. Infect Immun. 1990 Jun;58(6):1711–1719. doi: 10.1128/iai.58.6.1711-1719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yelton D. B., Limberger R. J., Curci K., Malinosky-Rummell F., Slivienski L., Schouls L. M., van Embden J. D., Charon N. W. Treponema phagedenis encodes and expresses homologs of the Treponema pallidum TmpA and TmpB proteins. Infect Immun. 1991 Oct;59(10):3685–3693. doi: 10.1128/iai.59.10.3685-3693.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijk R. V., Menke H. E., Tideman G. J., Stolz E. Enzyme linked immunosorbent assays with Treponema pallidum or axial filament of T phagedenis biotype Reiter as antigen: evaluation as screening tests for syphilis. Genitourin Med. 1986 Dec;62(6):367–372. doi: 10.1136/sti.62.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]