Abstract
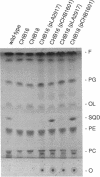
Two new mutants of Rhodobacter sphaeroides deficient in sulfolipid accumulation were isolated by directly screening mutagenized cell lines for polar lipid composition by thin-layer chromatography of lipid extracts. A genomic clone which complemented the mutations in these two lines, but not the previously described sulfolipid-deficient sqdA mutant, was identified. Sequence analysis of the relevant region of the clone revealed three, in tandem open reading frames, designated sqdB, ORF2, and sqdC. One of the mutants was complemented by the sqdB gene, and the other was complemented by the sqdC gene. Insertional inactivation of sqdB also inactivated sqdC, indicating that sqdB and sqdC are cotranscribed. The N-terminal region of the 46-kDa putative protein encoded by the sqdB gene showed slight homology to UDP-glucose epimerase from various organisms. The 30-kDa putative protein encoded by ORF2 showed very striking homology to rabbit muscle glycogenin, a UDP-glucose utilizing, autoglycosylating glycosyltransferase. The 26-kDa putative protein encoded by the sqdC gene was not homologous to any protein of known function.
Full text
PDF


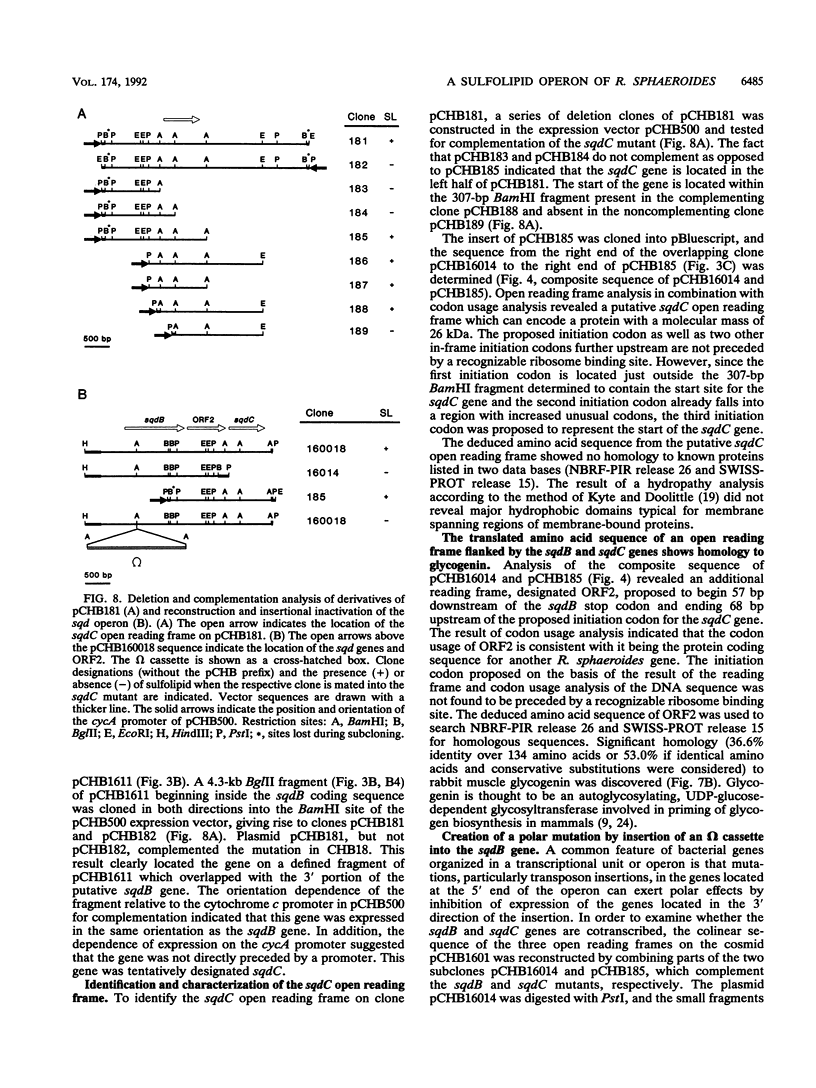


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. N., Hanson R. S. Construction of broad-host-range cosmid cloning vectors: identification of genes necessary for growth of Methylobacterium organophilum on methanol. J Bacteriol. 1985 Mar;161(3):955–962. doi: 10.1128/jb.161.3.955-962.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbás P., Soberón X., Merino E., Zurita M., Lomeli H., Valle F., Flores N., Bolivar F. Plasmid vector pBR322 and its special-purpose derivatives--a review. Gene. 1986;50(1-3):3–40. doi: 10.1016/0378-1119(86)90307-0. [DOI] [PubMed] [Google Scholar]
- Benning C., Somerville C. R. Isolation and genetic complementation of a sulfolipid-deficient mutant of Rhodobacter sphaeroides. J Bacteriol. 1992 Apr;174(7):2352–2360. doi: 10.1128/jb.174.7.2352-2360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell D. G., Cohen P. The amino acid sequence of rabbit skeletal muscle glycogenin. Eur J Biochem. 1989 Oct 20;185(1):119–125. doi: 10.1111/j.1432-1033.1989.tb15090.x. [DOI] [PubMed] [Google Scholar]
- Citron B. A., Donelson J. E. Sequence of the Saccharomyces GAL region and its transcription in vivo. J Bacteriol. 1984 Apr;158(1):269–278. doi: 10.1128/jb.158.1.269-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Schmidt H., Hoch M., Jung K. H., Binder H., Schmidt R. R. Synthesis of different nucleoside 5'-diphospho-sulfoquinovoses and their use for studies on sulfolipid biosynthesis in chloroplasts. Eur J Biochem. 1989 Sep 15;184(2):445–453. doi: 10.1111/j.1432-1033.1989.tb15037.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lemaire H. G., Müller-Hill B. Nucleotide sequences of the gal E gene and the gal T gene of E. coli. Nucleic Acids Res. 1986 Oct 10;14(19):7705–7711. doi: 10.1093/nar/14.19.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Parkison C., Cheng S. Y. A convenient method to increase the number of readable bases in DNA sequencing. Biotechniques. 1989 Sep;7(8):828–829. [PubMed] [Google Scholar]
- Pitcher J., Smythe C., Cohen P. Glycogenin is the priming glucosyltransferase required for the initiation of glycogen biogenesis in rabbit skeletal muscle. Eur J Biochem. 1988 Sep 15;176(2):391–395. doi: 10.1111/j.1432-1033.1988.tb14294.x. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Radunz A. Uber das Sulfochinovosyl-diacylglycerin aus höheren Pflanzen, Algen und Purpurbakterien. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):411–417. [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. The kinetics of the synthesis of photopigments in Rhodopseudomonas spheroides. J Gen Microbiol. 1962 Sep;28:607–616. doi: 10.1099/00221287-28-4-607. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]