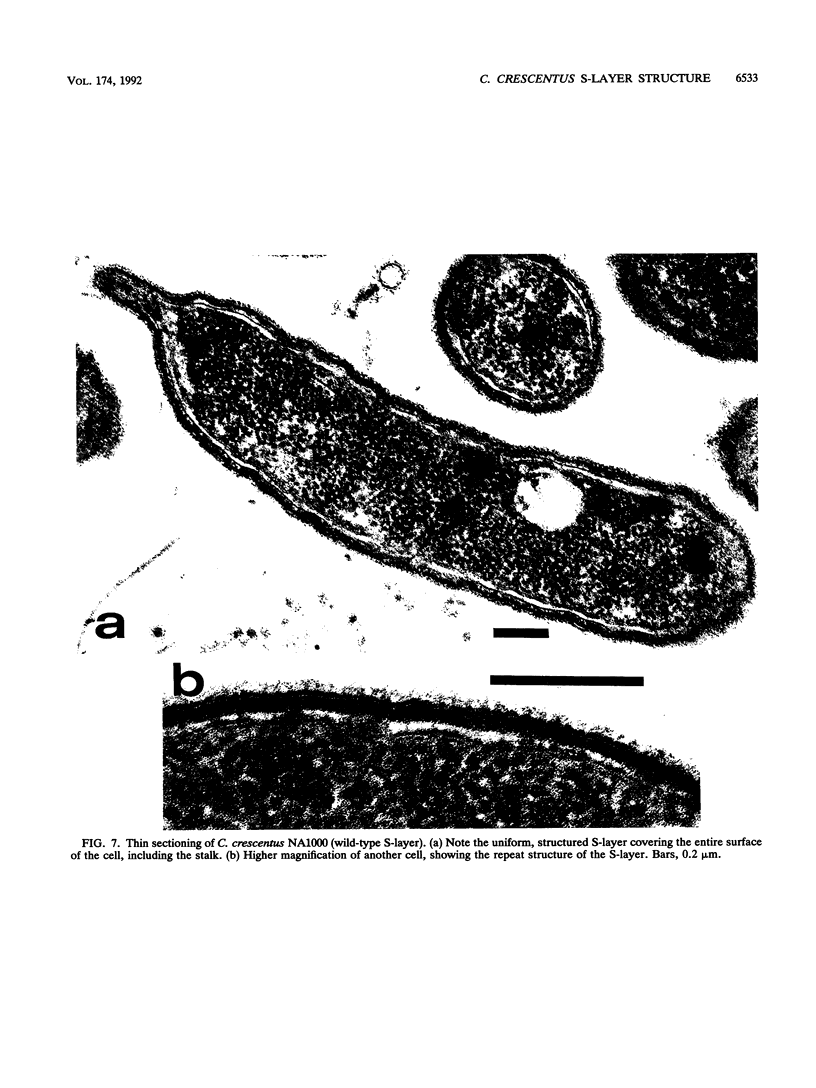
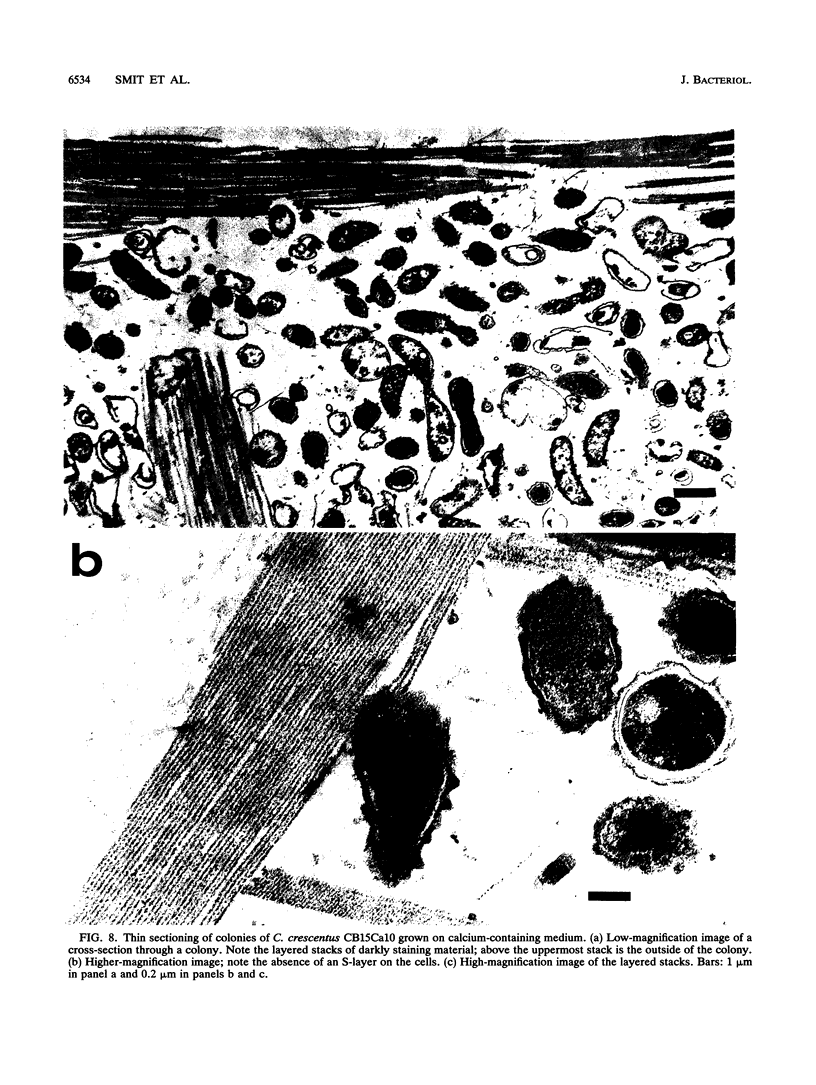
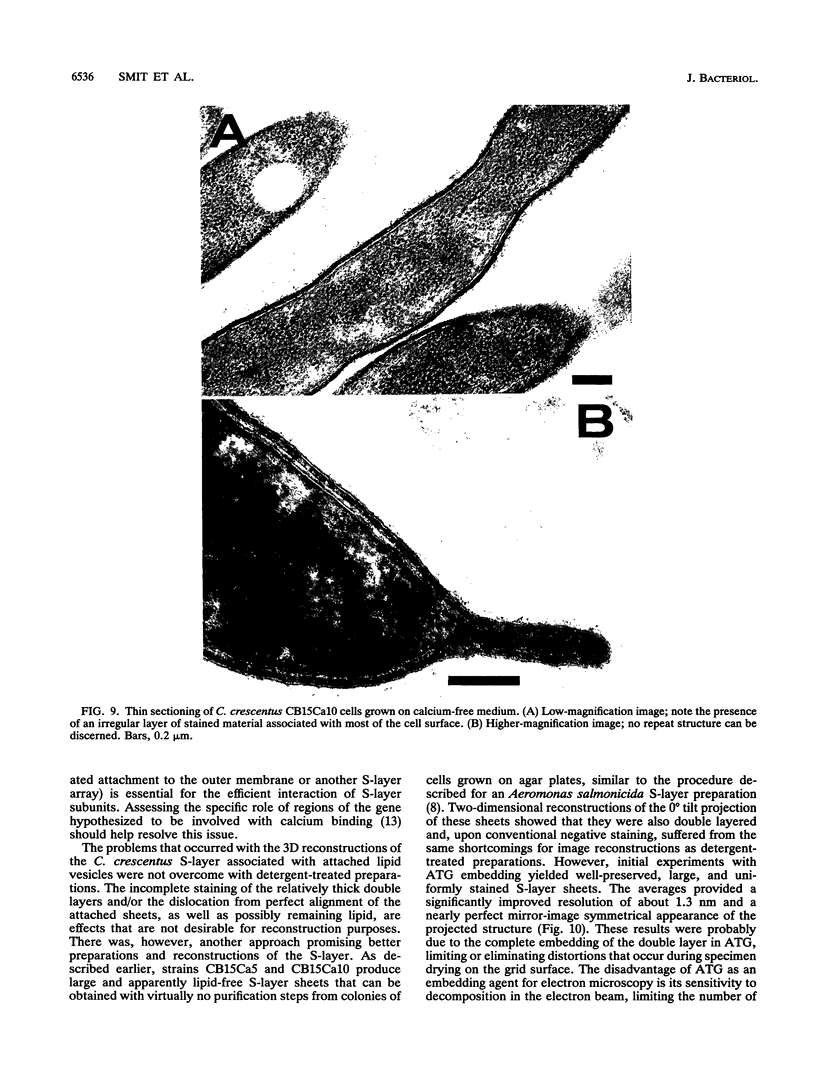
Abstract
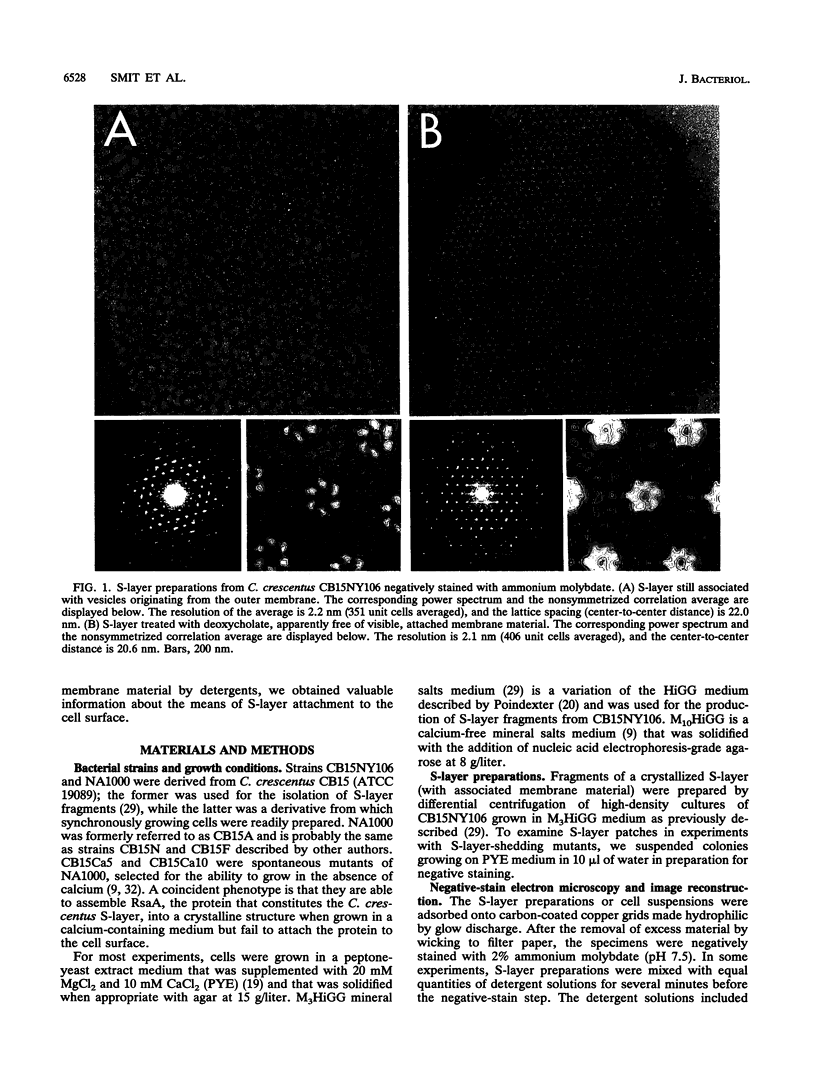
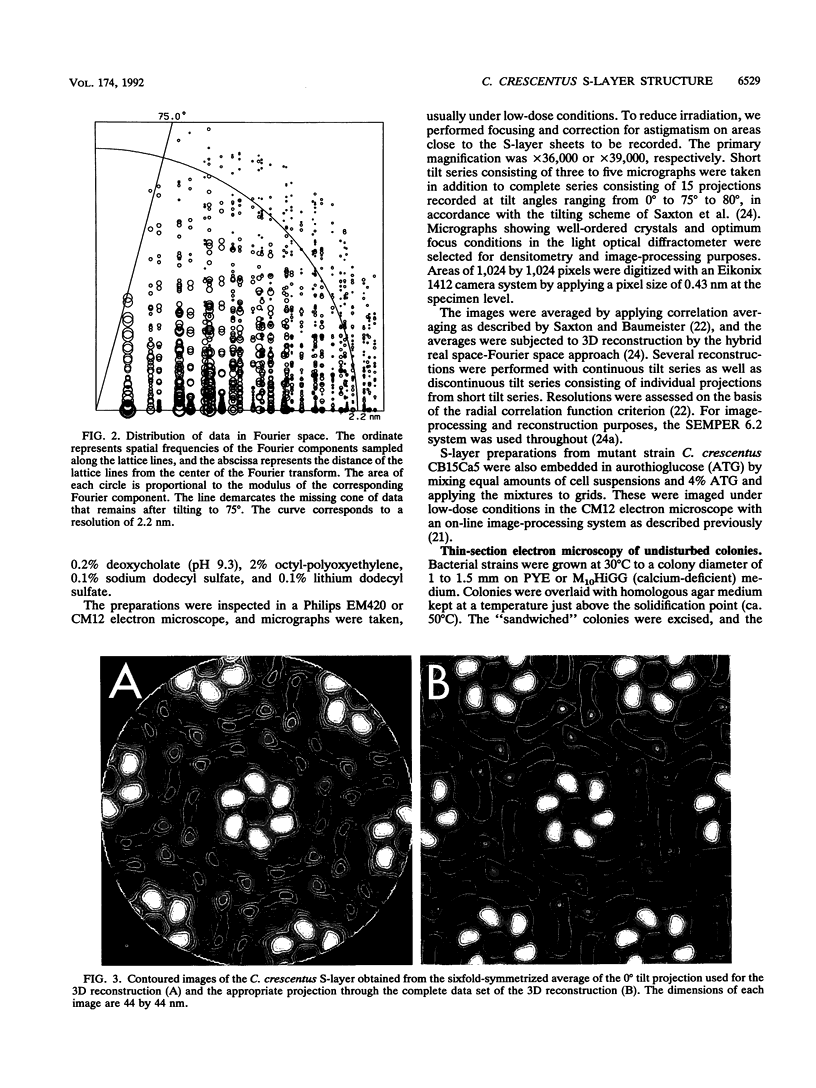
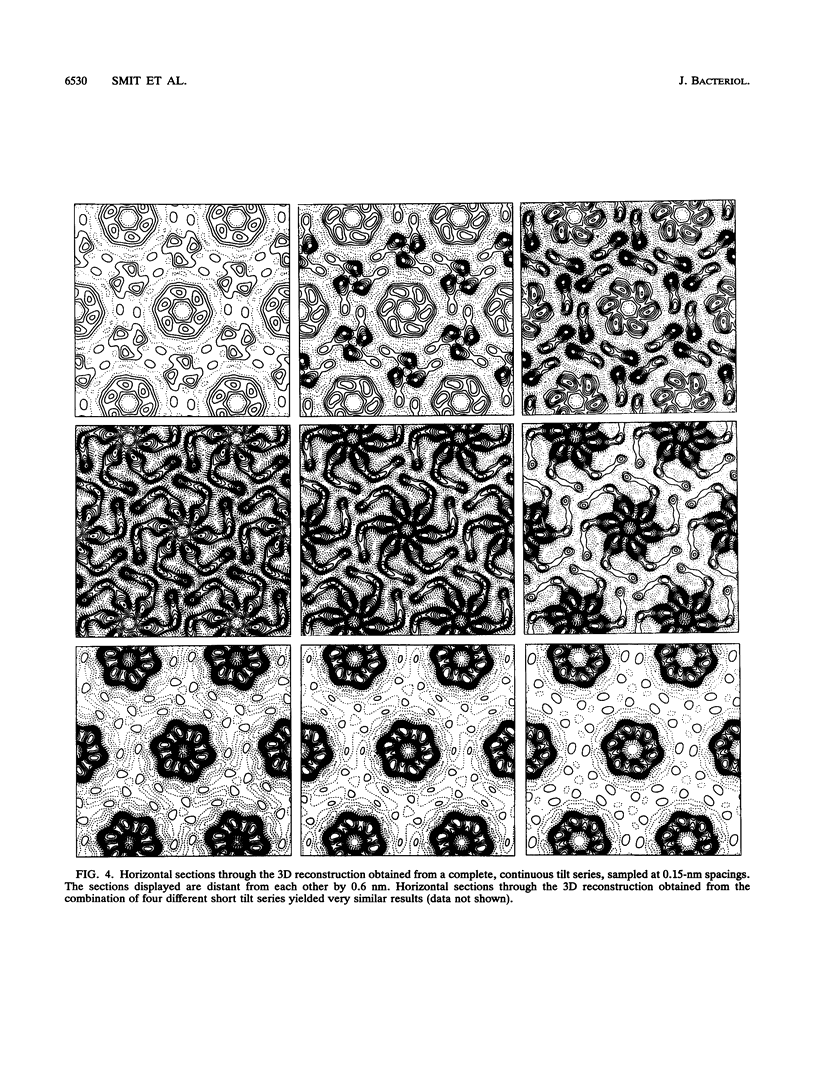
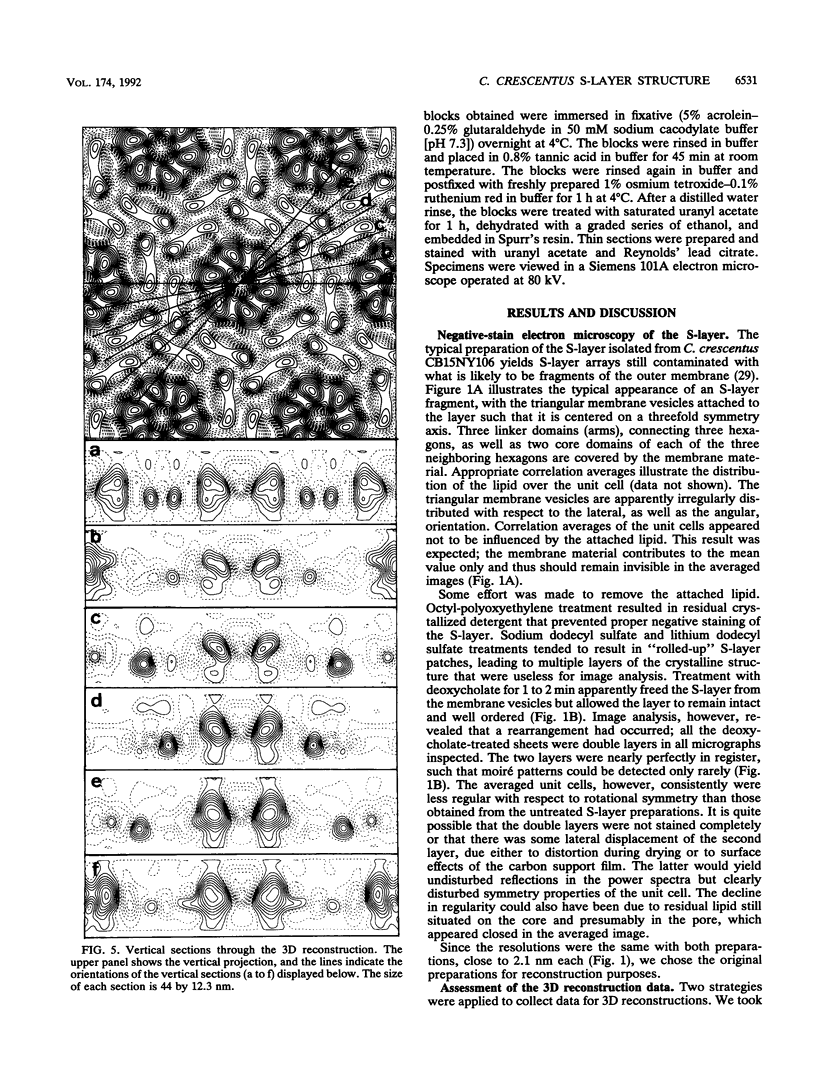
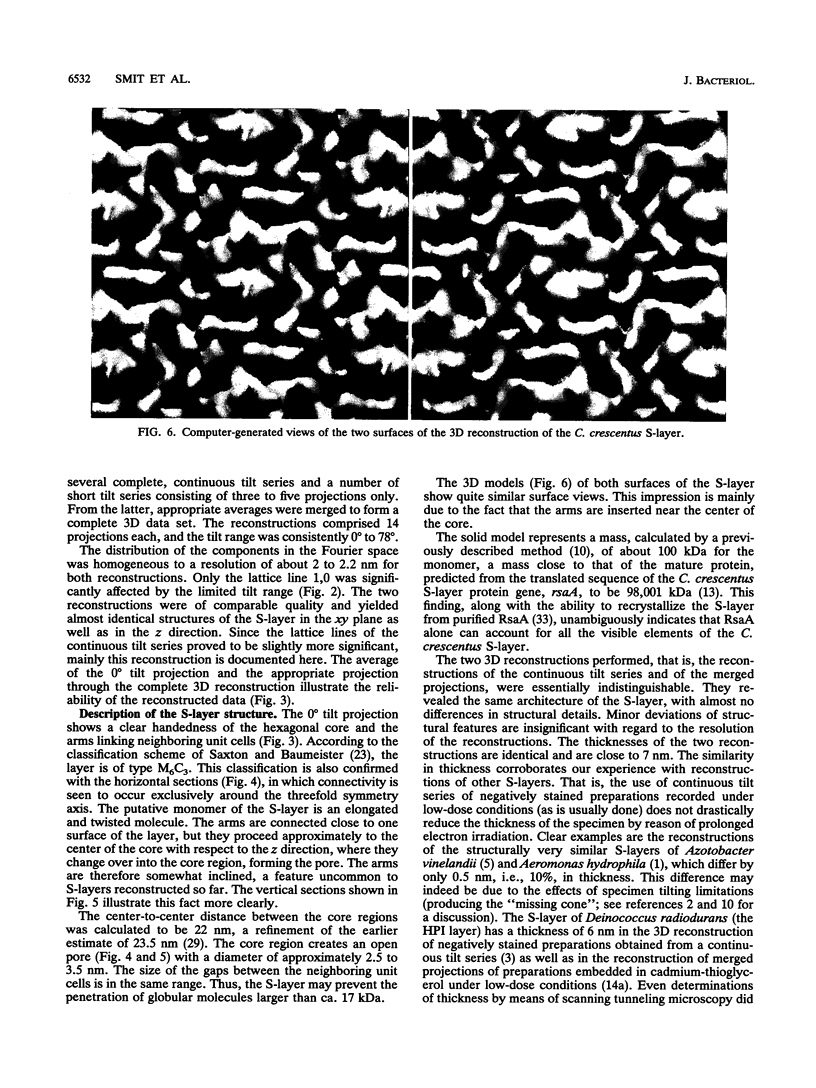
The regular surface protein structure (S-layer) of Caulobacter crescentus was analyzed by electron microscopy and three-dimensional image reconstruction to a resolution of 2 nm. Projections showed that the S-layer is an array of ring structures, each composed of six subunits that are arranged on a lattice with p6 symmetry. Three-dimensional reconstructions showed that the ring subunits were approximately rod-shaped structures and were perpendicular to the plane of the array, with a linker arm emanating from approximately the middle of the rod, accounting for the connections between the rings. The calculated subunit mass was ca. 100 kDa, very close to the size of RsaA (the protein known to be at least the predominant species in the S-layer) predicted from the DNA sequence of the rsaA gene. The core region of the rings creates an open pore 2.5 to 3.5 nm in diameter. The size of the gaps between the neighboring unit cells is in the same range, suggesting a uniform porosity predicted to exclude molecules larger than ca. 17 kDa. Attempts to remove membrane material from S-layer preparations with detergents revealed that the structure spontaneously rearranged into a mirror-image double layer. Negative-stain and thin-section electron microscopy examination of colonies of C. crescentus strains with a mutation in a surface molecule involved in the attachment of the S-layer showed that shed RsaA protein organized into large sheets. The sheets in turn organized into stacks that tended to accumulate near the upper surface of the colony. Image reconstruction indicated that these sheets were also precise mirror-image double layers, and thickness measurements obtained from thin sections were consistent with this finding. The sheets were absent when these mutant strains were grown without calcium, supporting other data that calcium is involved in attachment of the S-layer to a surface molecule and perhaps in subunit-subunit interactions. We propose that when the membrane is removed from S-layer fragments by detergents or the attachment-related surface molecule is absent, the attachment sites of the S-layer align precisely to form a double layer via a calcium interaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Karadaghi S., Wang D. N., Hovmöller S. Three-dimensional structure of the crystalline surface layer from Aeromonas hydrophila. J Ultrastruct Mol Struct Res. 1988 Oct;101(1):92–97. doi: 10.1016/0889-1605(88)90084-5. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Barth M., Hegerl R., Guckenberger R., Hahn M., Saxton W. O. Three-dimensional structure of the regular surface layer (HPI layer) of Deinococcus radiodurans. J Mol Biol. 1986 Jan 20;187(2):241–250. doi: 10.1016/0022-2836(86)90231-7. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Wildhaber I., Phipps B. M. Principles of organization in eubacterial and archaebacterial surface proteins. Can J Microbiol. 1989 Jan;35(1):215–227. doi: 10.1139/m89-034. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J., Graham L. L. Surface layers of bacteria. Microbiol Rev. 1991 Dec;55(4):684–705. doi: 10.1128/mr.55.4.684-705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingle W. H., Engelhardt H., Page W. J., Baumeister W. Three-dimensional structure of the regular tetragonal surface layer of Azotobacter vinelandii. J Bacteriol. 1987 Nov;169(11):5008–5015. doi: 10.1128/jb.169.11.5008-5015.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Gotschlich E. C. Surface array protein of Campylobacter fetus. Cloning and gene structure. J Biol Chem. 1990 Aug 25;265(24):14529–14535. [PubMed] [Google Scholar]
- Chu S., Cavaignac S., Feutrier J., Phipps B. M., Kostrzynska M., Kay W. W., Trust T. J. Structure of the tetragonal surface virulence array protein and gene of Aeromonas salmonicida. J Biol Chem. 1991 Aug 15;266(23):15258–15265. [PubMed] [Google Scholar]
- Dooley J. S., Engelhardt H., Baumeister W., Kay W. W., Trust T. J. Three-dimensional structure of an open form of the surface layer from the fish pathogen Aeromonas salmonicida. J Bacteriol. 1989 Jan;171(1):190–197. doi: 10.1128/jb.171.1.190-197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P., Smit J. A transducing bacteriophage for Caulobacter crescentus uses the paracrystalline surface layer protein as a receptor. J Bacteriol. 1991 Sep;173(17):5568–5572. doi: 10.1128/jb.173.17.5568-5572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. A., Smit J., Agabian N. Transcriptional analysis of the major surface array gene of Caulobacter crescentus. J Bacteriol. 1988 Oct;170(10):4706–4713. doi: 10.1128/jb.170.10.4706-4713.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A., Fisher J. A., Smit J. Nucleotide sequence analysis of the gene encoding the Caulobacter crescentus paracrystalline surface layer protein. Can J Microbiol. 1992 Mar;38(3):193–202. doi: 10.1139/m92-033. [DOI] [PubMed] [Google Scholar]
- Hovmöller S., Sjögren A., Wang D. N. The structure of crystalline bacterial surface layers. Prog Biophys Mol Biol. 1988;51(2):131–163. doi: 10.1016/0079-6107(88)90012-0. [DOI] [PubMed] [Google Scholar]
- Koval S. F., Hynes S. H. Effect of paracrystalline protein surface layers on predation by Bdellovibrio bacteriovorus. J Bacteriol. 1991 Apr;173(7):2244–2249. doi: 10.1128/jb.173.7.2244-2249.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Peters M., Lottspeich F., Baumeister W. S-layer protein gene of Acetogenium kivui: cloning and expression in Escherichia coli and determination of the nucleotide sequence. J Bacteriol. 1989 Nov;171(11):6307–6315. doi: 10.1128/jb.171.11.6307-6315.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Peters M., Lottspeich F., Schäfer W., Baumeister W. Nucleotide sequence analysis of the gene encoding the Deinococcus radiodurans surface protein, derived amino acid sequence, and complementary protein chemical studies. J Bacteriol. 1987 Nov;169(11):5216–5223. doi: 10.1128/jb.169.11.5216-5223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. Selection for nonbuoyant morphological mutants of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1141–1145. doi: 10.1128/jb.135.3.1141-1145.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W., Hahn M. Three-dimensional reconstruction of imperfect two-dimensional crystals. Ultramicroscopy. 1984;13(1-2):57–70. doi: 10.1016/0304-3991(84)90057-3. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. Principles of organization in S layers. J Mol Biol. 1986 Jan 20;187(2):251–253. doi: 10.1016/0022-2836(86)90232-9. [DOI] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Agabian N. Cloning of the major protein of the Caulobacter crescentus periodic surface layer: detection and characterization of the cloned peptide by protein expression assays. J Bacteriol. 1984 Dec;160(3):1137–1145. doi: 10.1128/jb.160.3.1137-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J., Grano D. A., Glaeser R. M., Agabian N. Periodic surface array in Caulobacter crescentus: fine structure and chemical analysis. J Bacteriol. 1981 Jun;146(3):1135–1150. doi: 10.1128/jb.146.3.1135-1150.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Adachi T., Sasaki T., Hayakawa S., Yamagata H., Tsukagoshi N., Udaka S. Characterization of the genes for the hexagonally arranged surface layer proteins in protein-producing Bacillus brevis 47: complete nucleotide sequence of the middle wall protein gene. J Bacteriol. 1988 Feb;170(2):935–945. doi: 10.1128/jb.170.2.935-945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Tabata R., Takahashi Y., Hashiba H., Sasaki T., Yamagata H., Tsukagoshi N., Udaka S. Characterization of the genes coding for two major cell wall proteins from protein-producing Bacillus brevis 47: complete nucleotide sequence of the outer wall protein gene. J Bacteriol. 1986 Oct;168(1):365–373. doi: 10.1128/jb.168.1.365-373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S. G., Smith S. H., Smit J. Isolation and comparison of the paracrystalline surface layer proteins of freshwater caulobacters. J Bacteriol. 1992 Mar;174(6):1783–1792. doi: 10.1128/jb.174.6.1783-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. H., Hartmann T., Baumeister W., Guckenberger R. Thickness determination of biological samples with a zeta-calibrated scanning tunneling microscope. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9343–9347. doi: 10.1073/pnas.87.23.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]