Abstract
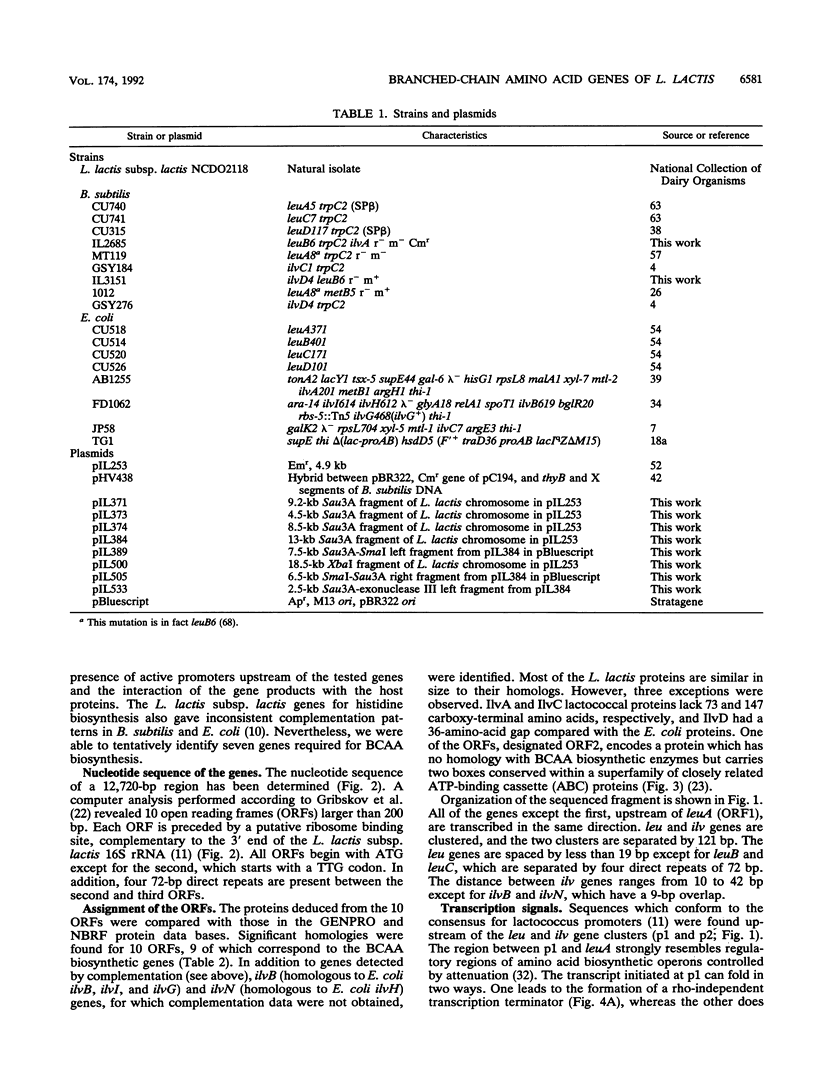
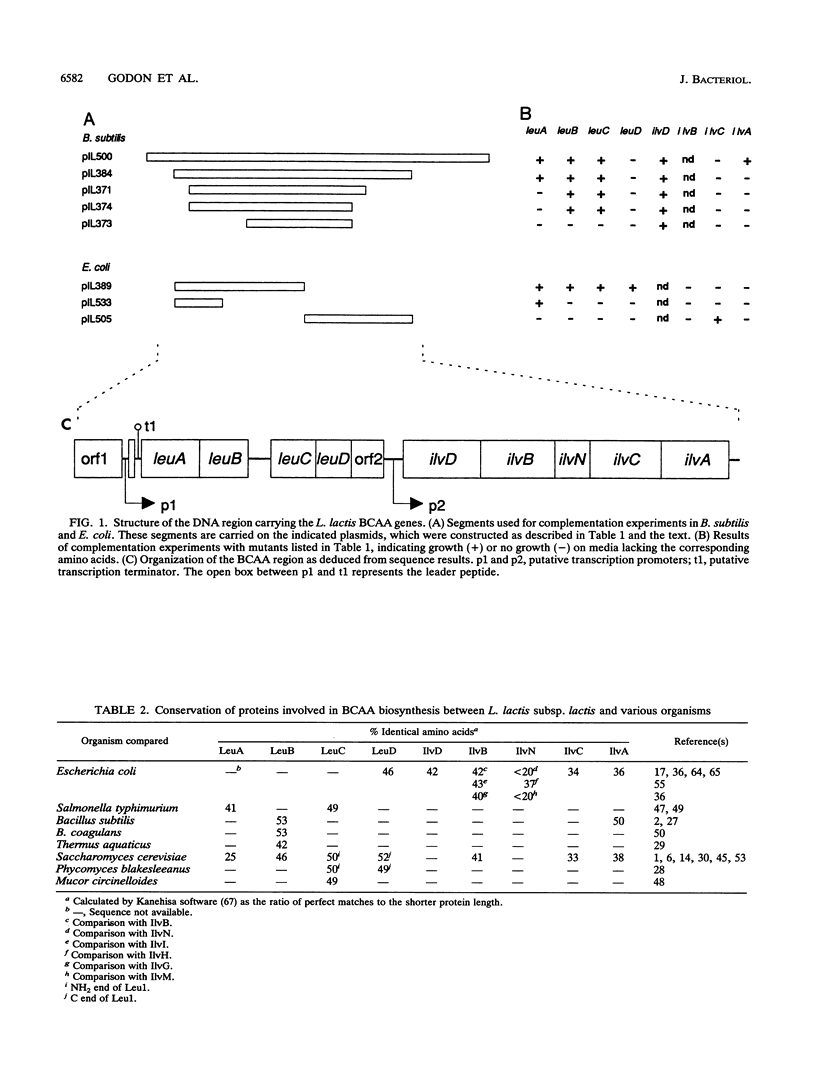
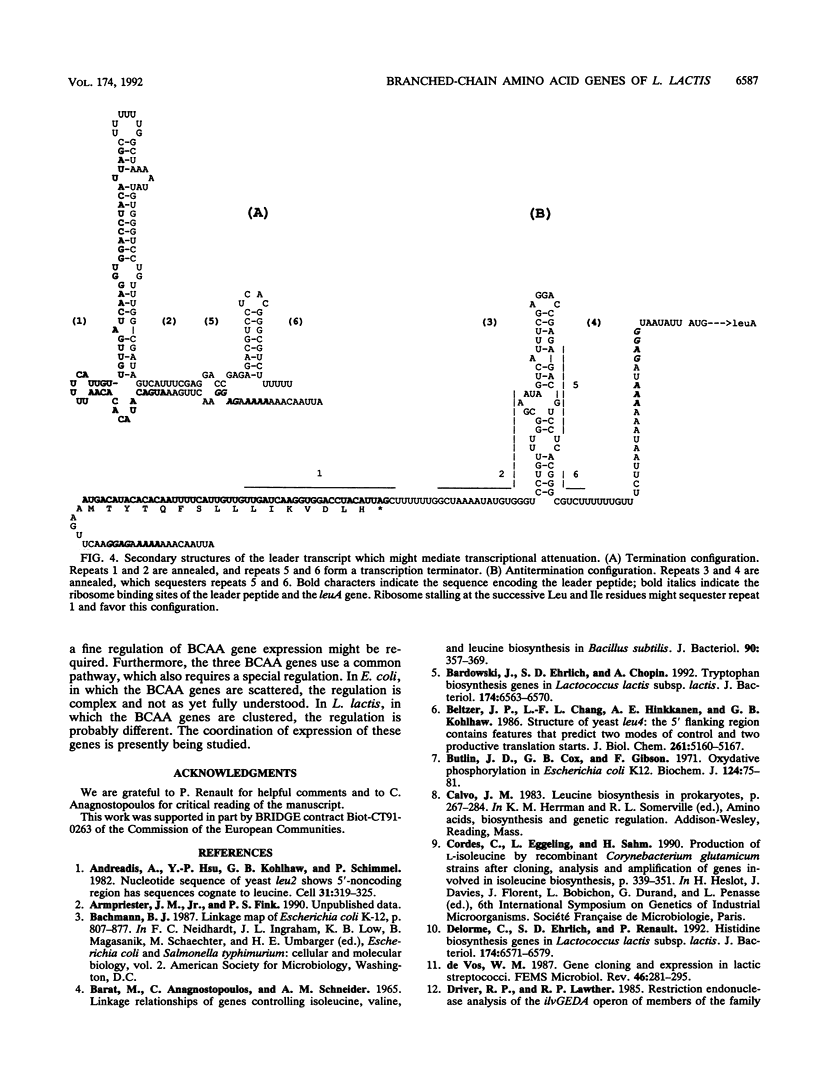
The genes for biosynthesis of the branched-chain amino acids leucine, isoleucine, and valine in Lactococcus lactis subsp. lactis NCDO2118 were characterized by cloning, complementation in Escherichia coli and Bacillus subtilis, and nucleotide sequence analysis. Nine structural genes are clustered on a 12-kb DNA fragment in the order leuABCD ilvDBNCA. Upstream of these genes, the nucleotide sequence suggests the existence of regulation by transcriptional attenuation. Between the leuD and ilvD genes is an unexpected gene, encoding a protein which belongs to the ATP-binding cassette protein superfamily.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- BARAT M., ANAGNOSTOPOULOS C., SCHNEIDER A. M. LINKAGE RELATIONSHIPS OF GENES CONTROLLING ISOLEUCINE, VALINE, AND LEUCINE BIOSYNTHESIS IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:357–369. doi: 10.1128/jb.90.2.357-369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardowski J., Ehrlich S. D., Chopin A. Tryptophan biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6563–6570. doi: 10.1128/jb.174.20.6563-6570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltzer J. P., Chang L. F., Hinkkanen A. E., Kohlhaw G. B. Structure of yeast LEU4. The 5' flanking region contains features that predict two modes of control and two productive translation starts. J Biol Chem. 1986 Apr 15;261(11):5160–5167. [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme C., Ehrlich S. D., Renault P. Histidine biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992 Oct;174(20):6571–6579. doi: 10.1128/jb.174.20.6571-6579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver R. P., Lawther R. P. Restriction endonuclease analysis of the ilvGEDA operon of members of the family Enterobacteriaceae. J Bacteriol. 1985 Jun;162(3):1317–1319. doi: 10.1128/jb.162.3.1317-1319.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans I. J., Downie J. A. The nodI gene product of Rhizobium leguminosarum is closely related to ATP-binding bacterial transport proteins; nucleotide sequence analysis of the nodI and nodJ genes. Gene. 1986;43(1-2):95–101. doi: 10.1016/0378-1119(86)90012-0. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friden P., Newman T., Freundlich M. Nucleotide sequence of the ilvB promoter-regulatory region: a biosynthetic operon controlled by attenuation and cyclic AMP. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6156–6160. doi: 10.1073/pnas.79.20.6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg D., Rosenthal E. R., Jones J. W., Calvo J. M. Characterization of the 3' end of the leucine operon of Salmonella typhimurium. Mol Gen Genet. 1985;199(3):486–494. doi: 10.1007/BF00330763. [DOI] [PubMed] [Google Scholar]
- Gemmill R. M., Wessler S. R., Keller E. B., Calvo J. M. leu operon of Salmonella typhimurium is controlled by an attenuation mechanism. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4941–4945. doi: 10.1073/pnas.76.10.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Nikaido H., Hofnung M. Sequence of the malK gene in E.coli K12. Nucleic Acids Res. 1982 Nov 25;10(22):7449–7458. doi: 10.1093/nar/10.22.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar J. Nucleotide sequence of the osmoregulatory proU operon of Escherichia coli. J Bacteriol. 1989 Apr;171(4):1923–1931. doi: 10.1128/jb.171.4.1923-1931.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribskov M., Devereux J., Burgess R. R. The codon preference plot: graphic analysis of protein coding sequences and prediction of gene expression. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):539–549. doi: 10.1093/nar/12.1part2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Gallagher M. P., Mimmack M. L., Pearce S. R. A family of closely related ATP-binding subunits from prokaryotic and eukaryotic cells. Bioessays. 1988 Apr;8(4):111–116. doi: 10.1002/bies.950080406. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Ikawa S., Shibata T., Ando T., Saito H. Genetic studies on site-specific endodeoxyribonucleases in Bacillus subtilis: multiple modification and restriction systems in transformants of Bacillus subtilis 168. Mol Gen Genet. 1980 Feb;177(3):359–368. doi: 10.1007/BF00271474. [DOI] [PubMed] [Google Scholar]
- Imai R., Sekiguchi T., Nosoh Y., Tsuda K. The nucleotide sequence of 3-isopropylmalate dehydrogenase gene from Bacillus subtilis. Nucleic Acids Res. 1987 Jun 25;15(12):4988–4988. doi: 10.1093/nar/15.12.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriaga E. A., Díaz-Mínguez J. M., Benito E. P., Alvarez M. I., Eslava A. P. Nucleotide sequence of the Phycomyces blakesleeanus leu1 gene. Nucleic Acids Res. 1990 Aug 11;18(15):4612–4612. doi: 10.1093/nar/18.15.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y., Nojima H., Nukiwa N., Ishizuka M., Nakajima T., Yasuhara T., Tanaka T., Oshima T. High guanine plus cytosine content in the third letter of codons of an extreme thermophile. DNA sequence of the isopropylmalate dehydrogenase of Thermus thermophilus. J Biol Chem. 1984 Mar 10;259(5):2956–2960. [PubMed] [Google Scholar]
- Kolter R., Yanofsky C. Attenuation in amino acid biosynthetic operons. Annu Rev Genet. 1982;16:113–134. doi: 10.1146/annurev.ge.16.120182.000553. [DOI] [PubMed] [Google Scholar]
- Kühnau S., Reyes M., Sievertsen A., Shuman H. A., Boos W. The activities of the Escherichia coli MalK protein in maltose transport, regulation, and inducer exclusion can be separated by mutations. J Bacteriol. 1991 Apr;173(7):2180–2186. doi: 10.1128/jb.173.7.2180-2186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Multivalent translational control of transcription termination at attenuator of ilvGEDA operon of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1862–1866. doi: 10.1073/pnas.77.4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Wek R. C., Lopes J. M., Pereira R., Taillon B. E., Hatfield G. W. The complete nucleotide sequence of the ilvGMEDA operon of Escherichia coli K-12. Nucleic Acids Res. 1987 Mar 11;15(5):2137–2155. doi: 10.1093/nar/15.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey C. J., Warburg R. J., Halvorson H. O., Zahler S. A. Genetic and physical analysis of the ilvBC-leu region in Bacillus subtilis. Gene. 1984 Dec;32(1-2):49–56. doi: 10.1016/0378-1119(84)90031-3. [DOI] [PubMed] [Google Scholar]
- Mackey C. J., Zahler S. A. Insertion of bacteriophage SP beta into the citF gene of Bacillus subtilis and specialized transduction of the ilvBC-leu genes. J Bacteriol. 1982 Sep;151(3):1222–1229. doi: 10.1128/jb.151.3.1222-1229.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh N. J., Duggan D. E. Ordering of mutant sites in the isoleucine-valine genes of Escherichia coli by use of merogenotes derived from F 14 : a new procedure for fine-structure mapping. J Bacteriol. 1972 Feb;109(2):730–740. doi: 10.1128/jb.109.2.730-740.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Niaudet B., Jannière L., Ehrlich S. D. Integration of linear, heterologous DNA molecules into the Bacillus subtilis chromosome: mechanism and use in induction of predictable rearrangements. J Bacteriol. 1985 Jul;163(1):111–120. doi: 10.1128/jb.163.1.111-120.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohno T., Saito T., Hong J. S. Cloning and complete nucleotide sequence of the Escherichia coli glutamine permease operon (glnHPQ). Mol Gen Genet. 1986 Nov;205(2):260–269. doi: 10.1007/BF00430437. [DOI] [PubMed] [Google Scholar]
- Pattee P. A. Genetic linkage of chromosomal tetracycline resistance and pigmentation to a purine auxotrophic marker and the isoleucine-valine-leucine structural genes in Staphylococcus aureus. J Bacteriol. 1976 Sep;127(3):1167–1172. doi: 10.1128/jb.127.3.1167-1172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. G., Holmberg S. The ILV5 gene of Saccharomyces cerevisiae is highly expressed. Nucleic Acids Res. 1986 Dec 22;14(24):9631–9651. doi: 10.1093/nar/14.24.9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J., Hoch J. A. Revised genetic linkage map of Bacillus subtilis. Microbiol Rev. 1985 Jun;49(2):158–179. doi: 10.1128/mr.49.2.158-179.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricca E., Calvo J. M. The nucleotide sequence of leuA from Salmonella typhimurium. Nucleic Acids Res. 1990 Mar 11;18(5):1290–1290. doi: 10.1093/nar/18.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncero M. I., Jepsen L. P., Strøman P., van Heeswijck R. Characterization of a leuA gene and an ARS element from Mucor circinelloides. Gene. 1989 Dec 14;84(2):335–343. doi: 10.1016/0378-1119(89)90508-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. R., Calvo J. M. The nucleotide sequence of leuC from Salmonella typhimurium. Nucleic Acids Res. 1990 May 25;18(10):3072–3072. doi: 10.1093/nar/18.10.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. M., Cowe E., Higgins D. G., Shields D. C., Wolfe K. H., Wright F. Codon usage patterns in Escherichia coli, Bacillus subtilis, Saccharomyces cerevisiae, Schizosaccharomyces pombe, Drosophila melanogaster and Homo sapiens; a review of the considerable within-species diversity. Nucleic Acids Res. 1988 Sep 12;16(17):8207–8211. doi: 10.1093/nar/16.17.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988 Apr;70(4):559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- Skala J., Capieaux E., Balzi E., Chen W. N., Goffeau A. Complete sequence of the Saccharomyces cerevisiae LEU1 gene encoding isopropylmalate isomerase. Yeast. 1991 Apr;7(3):281–285. doi: 10.1002/yea.320070310. [DOI] [PubMed] [Google Scholar]
- Somers J. M., Amzallag A., Middleton R. B. Genetic fine structure of the leucine operon of Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1268–1272. doi: 10.1128/jb.113.3.1268-1272.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Wessler S. R., Calvo J. M. Physical characterization of the ilvHI operon of Escherichia coli K-12. J Bacteriol. 1981 Sep;147(3):797–804. doi: 10.1128/jb.147.3.797-804.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Restriction of plasmid-mediated transformation in Bacillus subtilis 168. Mol Gen Genet. 1979 Sep;175(2):235–237. doi: 10.1007/BF00425542. [DOI] [PubMed] [Google Scholar]
- Ward J. B., Jr, Zahler S. A. Genetic studies of leucine biosynthesis in Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):719–726. doi: 10.1128/jb.116.2.719-726.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hatfield G. W. Nucleotide sequence and in vivo expression of the ilvY and ilvC genes in Escherichia coli K12. Transcription from divergent overlapping promoters. J Biol Chem. 1986 Feb 15;261(5):2441–2450. [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S. R., Calvo J. M. Control of leu operon expression in Escherichia coli by a transcription attenuation mechanism. J Mol Biol. 1981 Jul 15;149(4):579–597. doi: 10.1016/0022-2836(81)90348-x. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Guchte M., Kok J., Venema G. Gene expression in Lactococcus lactis. FEMS Microbiol Rev. 1992 Feb;8(2):73–92. doi: 10.1111/j.1574-6968.1992.tb04958.x. [DOI] [PubMed] [Google Scholar]


