Abstract
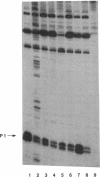
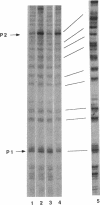
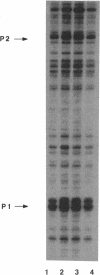
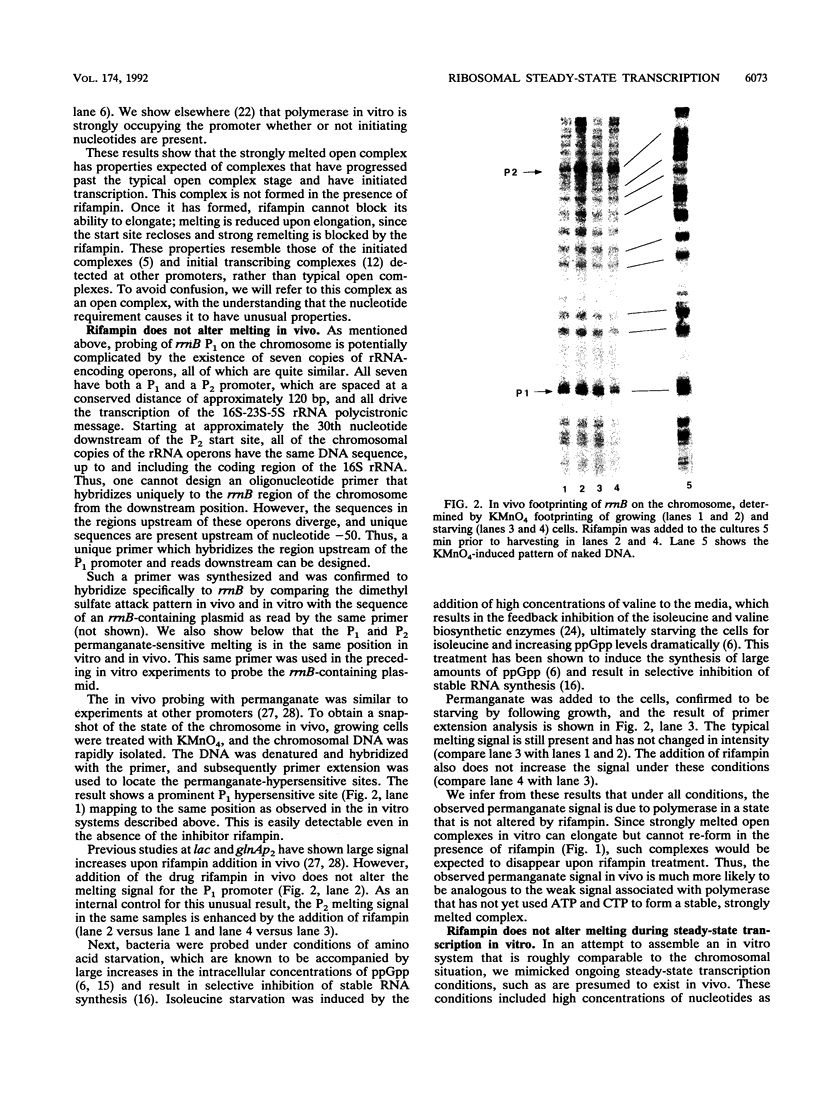
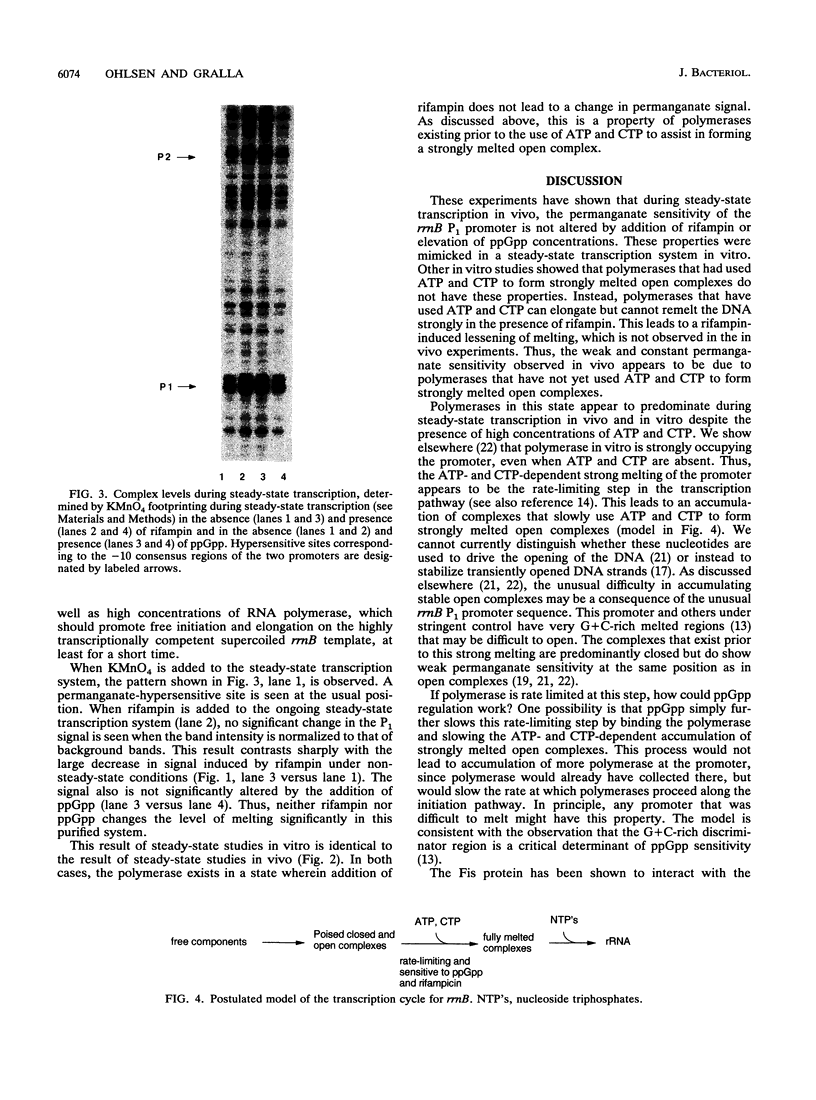
The rRNA rrnB P1 promoter was probed with the single-strand-selective reagent potassium permanganate during steady-state transcription in vitro and in vivo. In both cases, a weak but significant level of permanganate sensitivity was observed, which was not changed by treatment with rifampin. In contrast, static studies showed that rifampin strongly affects the very high level signal associated with polymerases that have used ATP and CTP as initiating nucleotides. We infer that the permanganate sensitivity associated with steady-state transcription is due to polymerases that have not yet used ATP and CTP. The slow and regulated step during rrnB P1 transcription may be the use of the initiating nucleotides to catalyze stable opening of the promoter DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baracchini E., Bremer H. Stringent and growth control of rRNA synthesis in Escherichia coli are both mediated by ppGpp. J Biol Chem. 1988 Feb 25;263(6):2597–2602. [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Carpousis A. J., Gralla J. D. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980 Jul 8;19(14):3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- Carpousis A. J., Gralla J. D. Interaction of RNA polymerase with lacUV5 promoter DNA during mRNA initiation and elongation. Footprinting, methylation, and rifampicin-sensitivity changes accompanying transcription initiation. J Mol Biol. 1985 May 25;183(2):165–177. doi: 10.1016/0022-2836(85)90210-4. [DOI] [PubMed] [Google Scholar]
- Cashel M., Gallant J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature. 1969 Mar 1;221(5183):838–841. doi: 10.1038/221838a0. [DOI] [PubMed] [Google Scholar]
- Gaal T., Gourse R. L. Guanosine 3'-diphosphate 5'-diphosphate is not required for growth rate-dependent control of rRNA synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5533–5537. doi: 10.1073/pnas.87.14.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L. Visualization and quantitative analysis of complex formation between E. coli RNA polymerase and an rRNA promoter in vitro. Nucleic Acids Res. 1988 Oct 25;16(20):9789–9809. doi: 10.1093/nar/16.20.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J. D. Promoter recognition and mRNA initiation by Escherichia coli E sigma 70. Methods Enzymol. 1990;185:37–54. doi: 10.1016/0076-6879(90)85006-a. [DOI] [PubMed] [Google Scholar]
- Krummel B., Chamberlin M. J. RNA chain initiation by Escherichia coli RNA polymerase. Structural transitions of the enzyme in early ternary complexes. Biochemistry. 1989 Sep 19;28(19):7829–7842. doi: 10.1021/bi00445a045. [DOI] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Stringent control of bacterial transcription. Cell. 1985 May;41(1):6–8. doi: 10.1016/0092-8674(85)90050-9. [DOI] [PubMed] [Google Scholar]
- Langert W., Meuthen M., Mueller K. Functional characteristics of the rrnD promoters of Escherichia coli. J Biol Chem. 1991 Nov 15;266(32):21608–21615. [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- Leirmo S., Gourse R. L. Factor-independent activation of Escherichia coli rRNA transcription. I. Kinetic analysis of the roles of the upstream activator region and supercoiling on transcription of the rrnB P1 promoter in vitro. J Mol Biol. 1991 Aug 5;220(3):555–568. doi: 10.1016/0022-2836(91)90100-k. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Newlands J. T., Ross W., Gosink K. K., Gourse R. L. Factor-independent activation of Escherichia coli rRNA transcription. II. characterization of complexes of rrnB P1 promoters containing or lacking the upstream activator region with Escherichia coli RNA polymerase. J Mol Biol. 1991 Aug 5;220(3):569–583. doi: 10.1016/0022-2836(91)90101-b. [DOI] [PubMed] [Google Scholar]
- Nilsson L., Vanet A., Vijgenboom E., Bosch L. The role of FIS in trans activation of stable RNA operons of E. coli. EMBO J. 1990 Mar;9(3):727–734. doi: 10.1002/j.1460-2075.1990.tb08166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Borman P. A rapid biochemical method for purifying high molecular weight bacterial chromosomal DNA for restriction enzyme analysis. Nucleic Acids Res. 1987 Apr 24;15(8):3631–3631. doi: 10.1093/nar/15.8.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Thompson J. F., Newlands J. T., Gourse R. L. E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990 Nov;9(11):3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. KMnO4 as a probe for lac promoter DNA melting and mechanism in vivo. J Biol Chem. 1989 May 15;264(14):8074–8081. [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Probing co-operative DNA-binding in vivo. The lac O1:O3 interaction. J Mol Biol. 1988 Jul 5;202(1):107–119. doi: 10.1016/0022-2836(88)90523-2. [DOI] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Probing the Escherichia coli glnALG upstream activation mechanism in vivo. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8934–8938. doi: 10.1073/pnas.85.23.8934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]