Abstract
Serratia marcescens exists in two cell forms and displays two kinds of motility depending on the type of growth surface encountered (L. Alberti and R. M. Harshey, J. Bacteriol. 172:4322-4328, 1990). In liquid medium, the bacteria are short rods with few flagella and show classical swimming behavior. Upon growth on a solid surface (0.7 to 0.85% agar), they differentiate into elongated, multinucleate, copiously flagellated forms that swarm over the agar surface. The flagella of swimmer and swarmer cells are composed of the same flagellin protein. We show in this study that disruption of hag, the gene encoding flagellin, abolishes both swimming and swarming motility. We have used transposon mini-Mu lac kan to isolate mutants of S. marcescens defective in both kinds of motility. Of the 155 mutants obtained, all Fla- mutants (lacking flagella) and Mot- mutants (paralyzed flagella) were defective for both swimming and swarming, as expected. All Che- mutants (chemotaxis defective) were also defective for swarming, suggesting that an intact chemotaxis system is essential for swarming. About one-third of the mutants were specifically affected only in swarming. Of this class, a large majority showed active "swarming motility" when viewed through the microscope (analogous to the active "swimming motility" of Che- mutants) but failed to show significant movement away from the site of initial inoculation on a macroscopic scale. These results suggest that bacteria swarming on a solid surface require many genes in addition to those required for chemotaxis and flagellar function, which extend the swarming movement outward. We also show in this study that nonflagellate S. marcescens is capable of spreading rapidly on low-agar media.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti L., Harshey R. M. Differentiation of Serratia marcescens 274 into swimmer and swarmer cells. J Bacteriol. 1990 Aug;172(8):4322–4328. doi: 10.1128/jb.172.8.4322-4328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T. K., Wasmuth C. R., Braunagel S. C., Benedik M. J. Expression of Serratia marcescens extracellular proteins requires recA. J Bacteriol. 1990 Jan;172(1):342–349. doi: 10.1128/jb.172.1.342-349.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Erskine D., Flaherty D. Proteus mirabilis mutants defective in swarmer cell differentiation and multicellular behavior. J Bacteriol. 1991 Oct;173(19):6279–6288. doi: 10.1128/jb.173.19.6279-6288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart B. D., Zusman D. R. "Frizzy" genes of Myxococcus xanthus are involved in control of frequency of reversal of gliding motility. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8767–8770. doi: 10.1073/pnas.82.24.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret R. B., Borkovich K. A., Simon M. I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
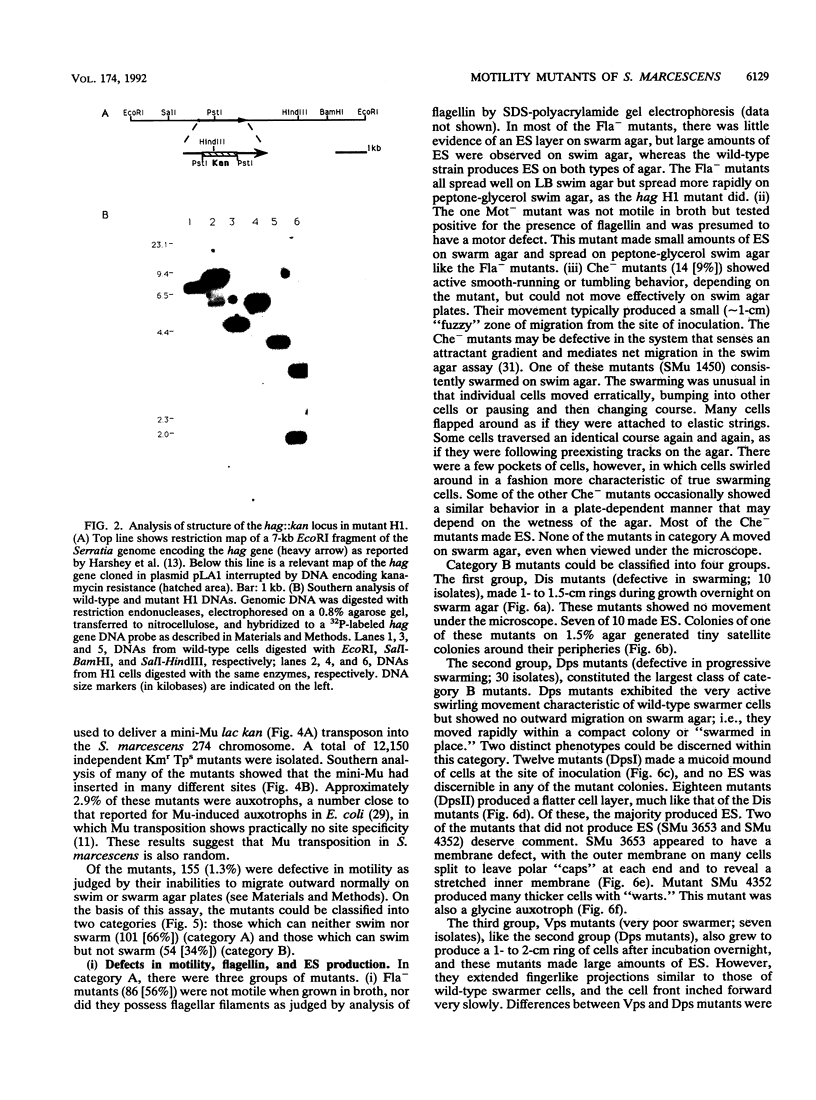
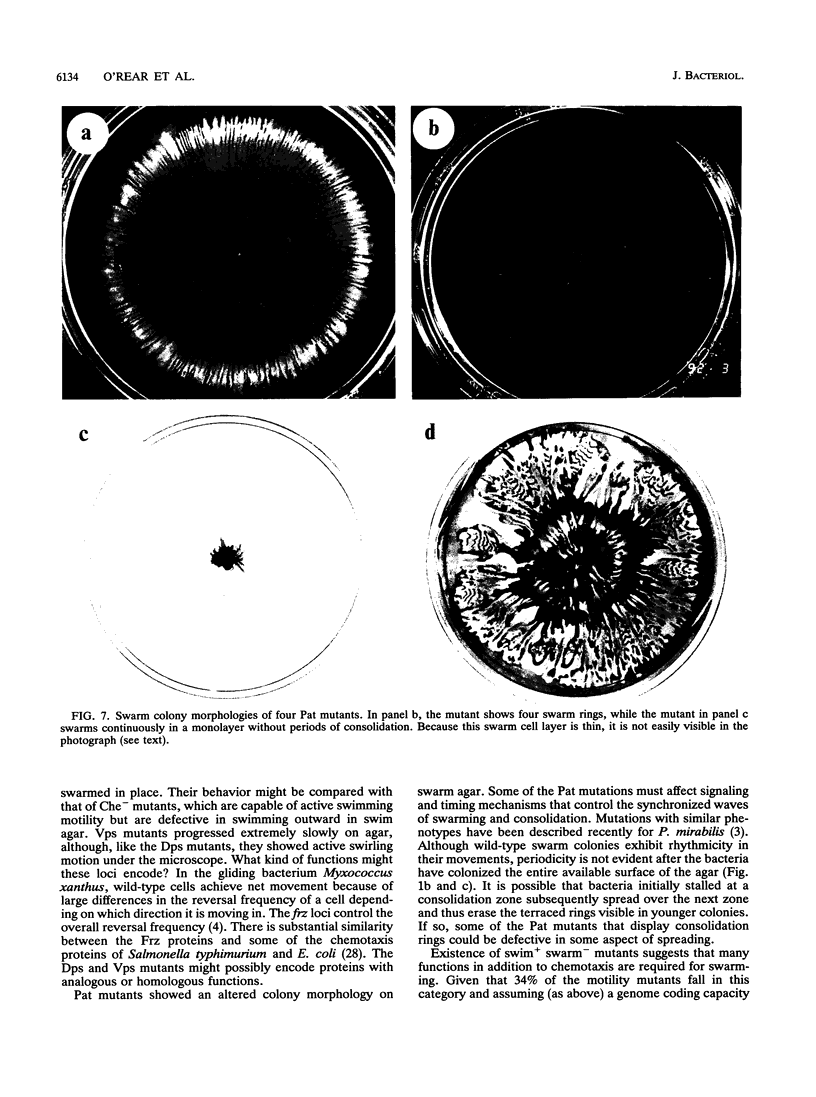
- Grimont P. A., Grimont F. The genus Serratia. Annu Rev Microbiol. 1978;32:221–248. doi: 10.1146/annurev.mi.32.100178.001253. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Casadaban M. J. Mini-mu bacteriophage with plasmid replicons for in vivo cloning and lac gene fusing. J Bacteriol. 1986 Oct;168(1):357–364. doi: 10.1128/jb.168.1.357-364.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshey R. M., Estepa G., Yanagi H. Cloning and nucleotide sequence of a flagellin-coding gene (hag) from Serratia marcescens 274. Gene. 1989 Jun 30;79(1):1–8. doi: 10.1016/0378-1119(89)90087-5. [DOI] [PubMed] [Google Scholar]
- Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972 Dec;36(4):478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines D. A., Saurugger P. N., Ihler G. M., Benedik M. J. Genetic analysis of extracellular proteins of Serratia marcescens. J Bacteriol. 1988 Sep;170(9):4141–4146. doi: 10.1128/jb.170.9.4141-4146.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häder D. P. Photosensory behavior in procaryotes. Microbiol Rev. 1987 Mar;51(1):1–21. doi: 10.1128/mr.51.1.1-21.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Kaneda K., Nakagawa Y., Isa K., Hara-Hotta H., Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992 Mar;174(6):1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Sogawa M., Nakagawa Y. Fractal spreading growth of Serratia marcescens which produces surface active exolipids. FEMS Microbiol Lett. 1989 Oct 15;52(3):243–246. doi: 10.1016/0378-1097(89)90204-8. [DOI] [PubMed] [Google Scholar]
- McCarter L., Hilmen M., Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988 Jul 29;54(3):345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- Paruchuri D. K., Harshey R. M. Flagellar variation in Serratia marcescens is associated with color variation. J Bacteriol. 1987 Jan;169(1):61–65. doi: 10.1128/jb.169.1.61-65.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sar N., McCarter L., Simon M., Silverman M. Chemotactic control of the two flagellar systems of Vibrio parahaemolyticus. J Bacteriol. 1990 Jan;172(1):334–341. doi: 10.1128/jb.172.1.334-341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990 Dec;54(4):473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]









