Abstract
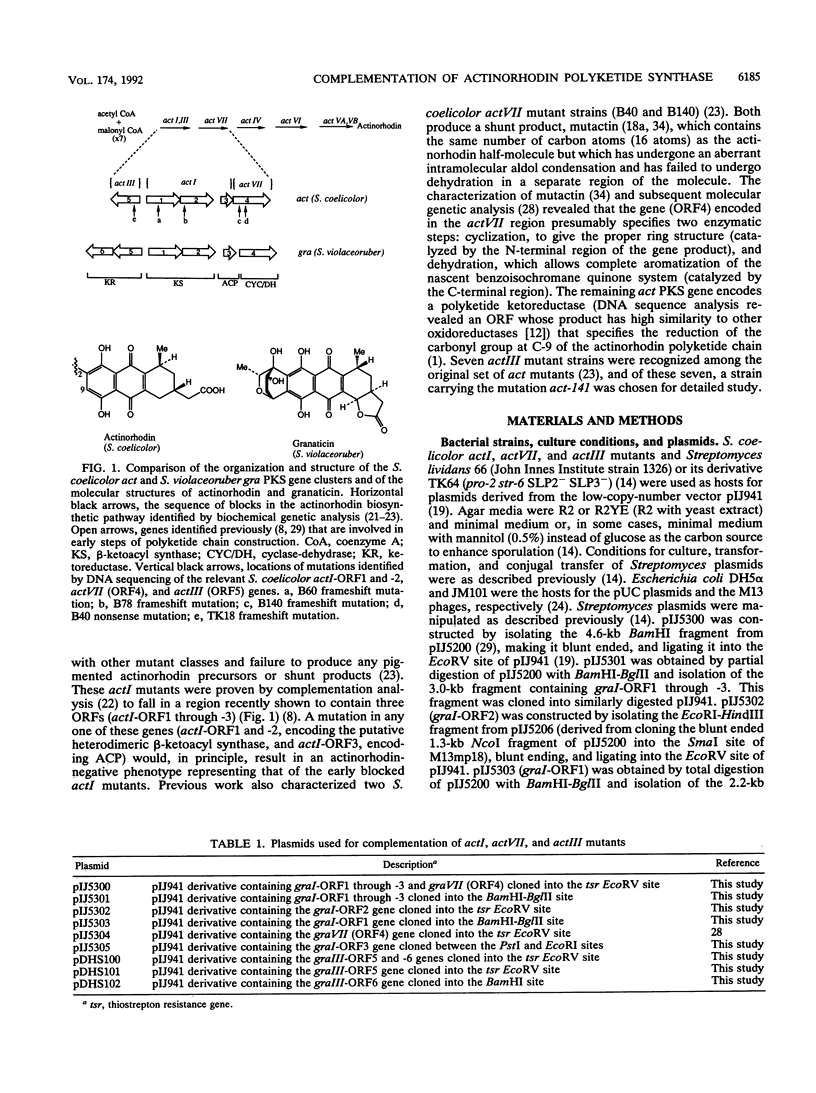
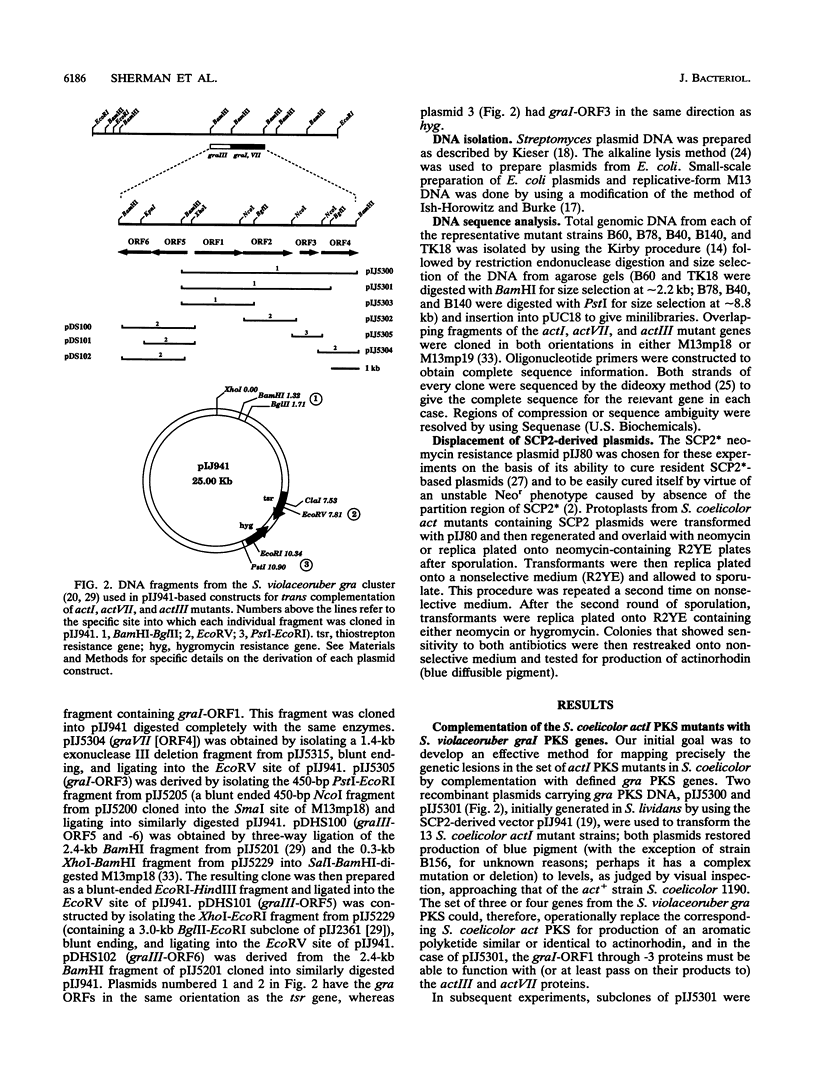
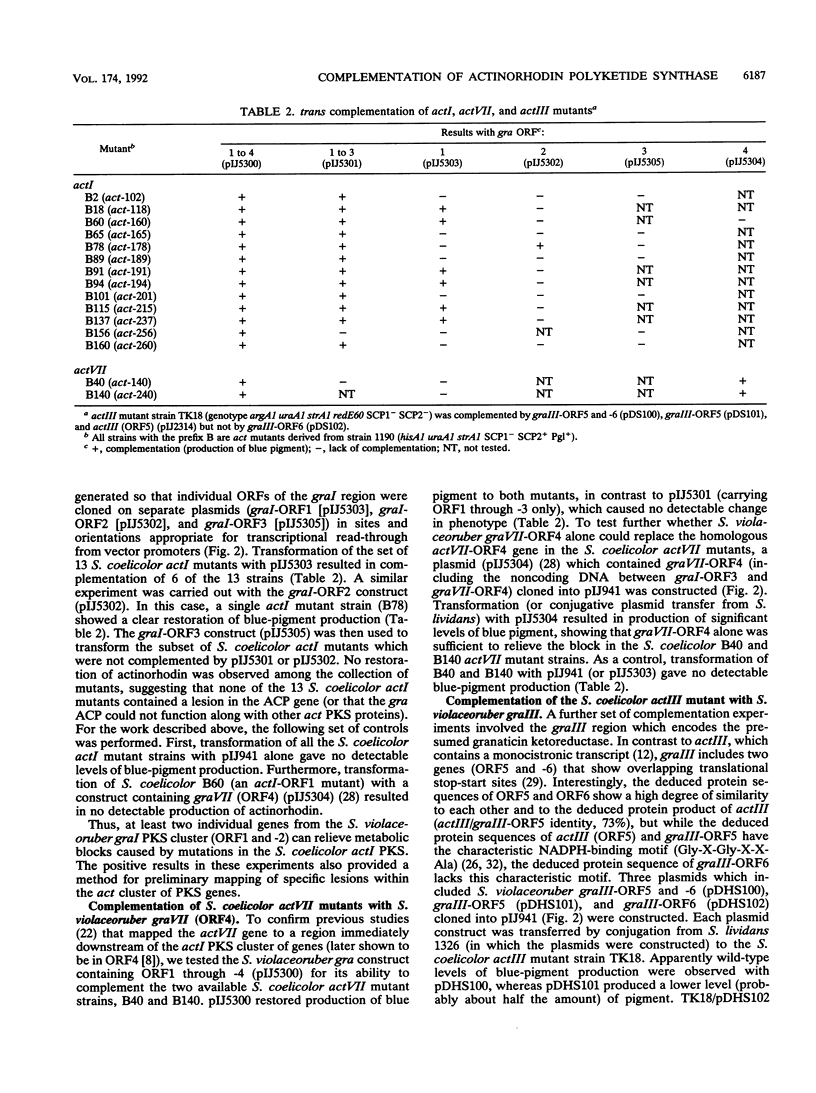
Streptomyces coelicolor A3(2) and Streptomyces violaceoruber Tü22 produce the antibiotics actinorhodin and granaticin, respectively. Both the aglycone of granaticin and the half-molecule of actinorhodin are derived from one acetyl coenzyme A starter unit and seven malonyl coenzyme A extender units via the polyketide pathway to produce benzoisochromane quinone moieties with identical structures (except for the stereochemistry at two chiral centers). In S. coelicolor and S. violaceoruber, the type II polyketide synthase (PKS) is encoded by clusters of five and six genes, respectively. We complemented a series of S. coelicolor mutants (act) defective in different components of the PKS (actI for carbon chain assembly, actIII for ketoreduction, and actVII for cyclization-dehydration) by the corresponding genes (gra) from S. violaceoruber introduced in trans on low-copy-number plasmids. This procedure showed that four of the act PKS components could be replaced by a heterologous gra protein to give a functional PKS. The analysis also served to identify which of three candidate open reading frames (ORFs) in the actI region had been altered in each of a set of 13 actI mutants. It also proved that actI-ORF2 (whose putative protein product shows overall similarity to the beta-ketoacyl synthase encoded by actI-ORF1 but whose function is unclear) is essential for PKS function. Mutations in each of the four complemented act genes (actI-ORF1, actI-ORF2, actIII, and actVII) were cloned and sequenced, revealing a nonsense or frameshift mutation in each mutant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartel P. L., Zhu C. B., Lampel J. S., Dosch D. C., Connors N. C., Strohl W. R., Beale J. M., Jr, Floss H. G. Biosynthesis of anthraquinones by interspecies cloning of actinorhodin biosynthesis genes in streptomycetes: clarification of actinorhodin gene functions. J Bacteriol. 1990 Sep;172(9):4816–4826. doi: 10.1128/jb.172.9.4816-4826.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb M. J., Ward J. M., Hopwood D. A. Transformation of plasmid DNA into Streptomyces at high frequency. Nature. 1978 Jul 27;274(5669):398–400. doi: 10.1038/274398a0. [DOI] [PubMed] [Google Scholar]
- Birch A. J. Biosynthesis of polyketides and related compounds. Science. 1967 Apr 14;156(3772):202–206. doi: 10.1126/science.156.3772.202. [DOI] [PubMed] [Google Scholar]
- Cortes J., Haydock S. F., Roberts G. A., Bevitt D. J., Leadlay P. F. An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature. 1990 Nov 8;348(6297):176–178. doi: 10.1038/348176a0. [DOI] [PubMed] [Google Scholar]
- Donadio S., Staver M. J., McAlpine J. B., Swanson S. J., Katz L. Modular organization of genes required for complex polyketide biosynthesis. Science. 1991 May 3;252(5006):675–679. doi: 10.1126/science.2024119. [DOI] [PubMed] [Google Scholar]
- Fernández-Moreno M. A., Caballero J. L., Hopwood D. A., Malpartida F. The act cluster contains regulatory and antibiotic export genes, direct targets for translational control by the bldA tRNA gene of Streptomyces. Cell. 1991 Aug 23;66(4):769–780. doi: 10.1016/0092-8674(91)90120-n. [DOI] [PubMed] [Google Scholar]
- Hallam S. E., Malpartida F., Hopwood D. A. Nucleotide sequence, transcription and deduced function of a gene involved in polyketide antibiotic synthesis in Streptomyces coelicolor. Gene. 1988 Dec 30;74(2):305–320. doi: 10.1016/0378-1119(88)90165-5. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Malpartida F., Kieser H. M., Ikeda H., Duncan J., Fujii I., Rudd B. A., Floss H. G., Omura S. Production of 'hybrid' antibiotics by genetic engineering. Nature. 1985 Apr 18;314(6012):642–644. doi: 10.1038/314642a0. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Sherman D. H. Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet. 1990;24:37–66. doi: 10.1146/annurev.ge.24.120190.000345. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Lydiate D. J., Malpartida F., Hopwood D. A. The Streptomyces plasmid SCP2*: its functional analysis and development into useful cloning vectors. Gene. 1985;35(3):223–235. doi: 10.1016/0378-1119(85)90001-0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hallam S. E., Kieser H. M., Motamedi H., Hutchinson C. R., Butler M. J., Sugden D. A., Warren M., McKillop C., Bailey C. R. Homology between Streptomyces genes coding for synthesis of different polyketides used to clone antibiotic biosynthetic genes. 1987 Feb 26-Mar 4Nature. 325(6107):818–821. doi: 10.1038/325818a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Physical and genetic characterisation of the gene cluster for the antibiotic actinorhodin in Streptomyces coelicolor A3(2). Mol Gen Genet. 1986 Oct;205(1):66–73. doi: 10.1007/BF02428033. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrutton N. S., Berry A., Perham R. N. Redesign of the coenzyme specificity of a dehydrogenase by protein engineering. Nature. 1990 Jan 4;343(6253):38–43. doi: 10.1038/343038a0. [DOI] [PubMed] [Google Scholar]
- Sherman D. H., Malpartida F., Bibb M. J., Kieser H. M., Bibb M. J., Hopwood D. A. Structure and deduced function of the granaticin-producing polyketide synthase gene cluster of Streptomyces violaceoruber Tü22. EMBO J. 1989 Sep;8(9):2717–2725. doi: 10.1002/j.1460-2075.1989.tb08413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Hol W. G. Predicted nucleotide-binding properties of p21 protein and its cancer-associated variant. Nature. 1983 Apr 28;302(5911):842–844. doi: 10.1038/302842a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


