Abstract
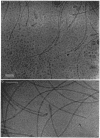
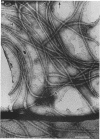
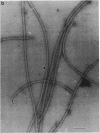
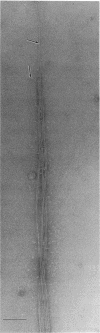
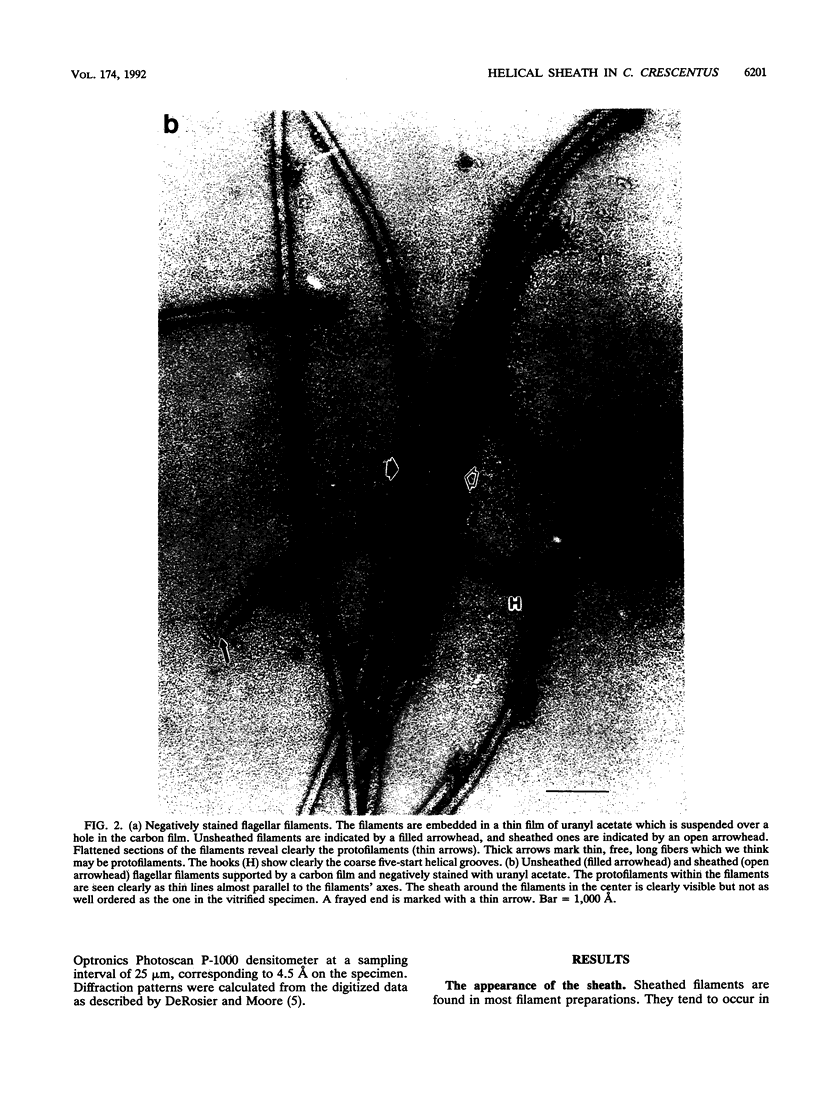
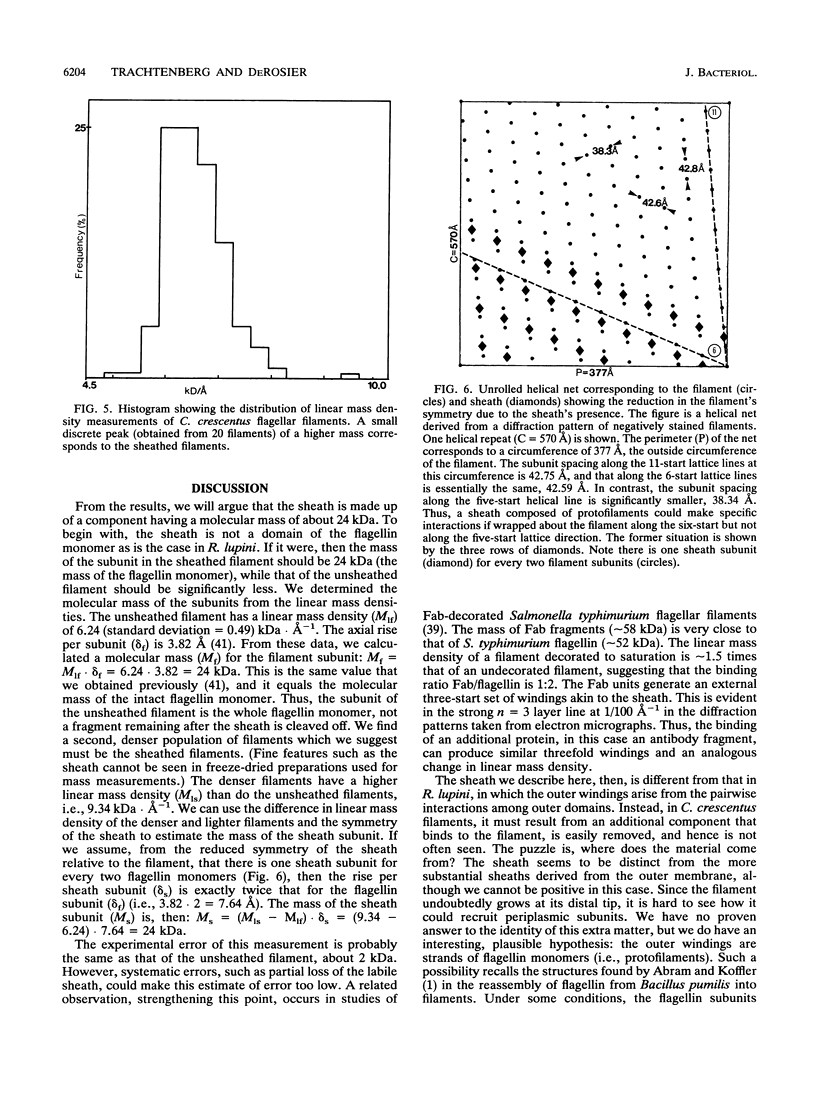
An unusual feature in preparations of the Caulobacter crescentus flagellar filaments is that some filaments are surrounded by a set of three windings that form a sheath. We provide evidence that the sheath is composed of subunits having a molecular mass of 24,000 Da. We suggest that the sheath could be composed of protofilaments of flagellin wound around the filament.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D., KOFFLER H. IN VITRO FORMATION OF FLAGELLA-LIKE FILAMENTS AND OTHER STRUCTURES FROM FLAGELLIN. J Mol Biol. 1964 Jul;9:168–185. doi: 10.1016/s0022-2836(64)80098-x. [DOI] [PubMed] [Google Scholar]
- Abram D., Vatter A. E., Koffler H. Attachment and structural features of flagella of certain bacilli. J Bacteriol. 1966 May;91(5):2045–2068. doi: 10.1128/jb.91.5.2045-2068.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Goldstein S. F., Curci K., Limberger R. J. The bent-end morphology of Treponema phagedenis is associated with short, left-handed, periplasmic flagella. J Bacteriol. 1991 Aug;173(15):4820–4826. doi: 10.1128/jb.173.15.4820-4826.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosier D. J., Moore P. B. Reconstruction of three-dimensional images from electron micrographs of structures with helical symmetry. J Mol Biol. 1970 Sep 14;52(2):355–369. doi: 10.1016/0022-2836(70)90036-7. [DOI] [PubMed] [Google Scholar]
- Driks A., Bryan R., Shapiro L., DeRosier D. J. The organization of the Caulobacter crescentus flagellar filament. J Mol Biol. 1989 Apr 20;206(4):627–636. doi: 10.1016/0022-2836(89)90571-8. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., KERRIDGE D., HORNE R. W. THE FINE STRUCTURE AND MODE OF ATTACHMENT OF THE SHEATHED FLAGELLUM OF VIBRIO METCHNIKOVII. J Cell Biol. 1963 Aug;18:327–336. doi: 10.1083/jcb.18.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill P. R., Agabian N. The nucleotide sequence of the Mr = 28,500 flagellin gene of Caulobacter crescentus. J Biol Chem. 1983 Jun 25;258(12):7395–7401. [PubMed] [Google Scholar]
- Hainfeld J. F., Wall J. S., Desmond E. J. A small computer system for micrograph analysis. Ultramicroscopy. 1982;8(3):263–270. doi: 10.1016/0304-3991(82)90242-x. [DOI] [PubMed] [Google Scholar]
- Hranitzky K. W., Mulholland A., Larson A. D., Eubanks E. R., Hart L. T. Characterization of a flagellar sheath protein of Vibrio cholerae. Infect Immun. 1980 Feb;27(2):597–603. doi: 10.1128/iai.27.2.597-603.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman H. C., Trachtenberg S. Point mutations that lock Salmonella typhimurium flagellar filaments in the straight right-handed and left-handed forms and their relation to filament superhelicity. J Mol Biol. 1991 Jul 5;220(1):79–88. doi: 10.1016/0022-2836(91)90382-g. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Ferber D. M., Ely B. Synthesis and assembly of flagellar components by Caulobacter crescentus motility mutants. J Bacteriol. 1983 Jun;154(3):1137–1144. doi: 10.1128/jb.154.3.1137-1144.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanto S., Okino H., Aizawa S., Yamaguchi S. Amino acids responsible for flagellar shape are distributed in terminal regions of flagellin. J Mol Biol. 1991 Jun 5;219(3):471–480. doi: 10.1016/0022-2836(91)90187-b. [DOI] [PubMed] [Google Scholar]
- Krupski G., Götz R., Ober K., Pleier E., Schmitt R. Structure of complex flagellar filaments in Rhizobium meliloti. J Bacteriol. 1985 Apr;162(1):361–366. doi: 10.1128/jb.162.1.361-366.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima G. Construction of a minimum-size functional flagellin of Escherichia coli. J Bacteriol. 1988 Jul;170(7):3305–3309. doi: 10.1128/jb.170.7.3305-3309.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWY J., HANSON J. ELECTRON MICROSCOPE STUDIES OF BACTERIAL FLAGELLA. J Mol Biol. 1965 Feb;11:293–313. doi: 10.1016/s0022-2836(65)80059-6. [DOI] [PubMed] [Google Scholar]
- LOWY J., HANSON J. STRUCTURE OF BACTERIAL FLAGELLA. Nature. 1964 May 9;202:538–540. doi: 10.1038/202538a0. [DOI] [PubMed] [Google Scholar]
- Lagenaur C., Agabian N. Physical characterization of Caulobacter crescentus flagella. J Bacteriol. 1976 Oct;128(1):435–444. doi: 10.1128/jb.128.1.435-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M. Comparison of the flagellins from different flagellar morphotypes of Escherichia coli. J Gen Microbiol. 1977 Jul;101(1):112–130. doi: 10.1099/00221287-101-1-121. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Orskov I., Orskov F. Morphological distinction between different H serotypes of Escherichia coli. J Gen Microbiol. 1977 Jul;101(1):111–119. doi: 10.1099/00221287-101-1-111. [DOI] [PubMed] [Google Scholar]
- Lotz W., Acker G., Schmitt R. Bacteriophage 7-7-1 adsorbs to the complex flagella of Rhizobium lupini H13-3. J Gen Virol. 1977 Jan;34(1):9–17. doi: 10.1099/0022-1317-34-1-9. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Lodderstaedt G., Schmitt R. Purification and biochemical properties of complex flagella isolated from Rhizobium lupini H13-3. Biochim Biophys Acta. 1978 Jul 21;535(1):110–124. doi: 10.1016/0005-2795(78)90038-7. [DOI] [PubMed] [Google Scholar]
- Meadows P. S. The attachment of bacteria to solid surfaces. Arch Mikrobiol. 1971;75(4):374–381. doi: 10.1007/BF00407699. [DOI] [PubMed] [Google Scholar]
- Minnich S. A., Newton A. Promoter mapping and cell cycle regulation of flagellin gene transcription in Caulobacter crescentus. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1142–1146. doi: 10.1073/pnas.84.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba K., Yamashita I., Vonderviszt F. Structure of the core and central channel of bacterial flagella. Nature. 1989 Dec 7;342(6250):648–654. doi: 10.1038/342648a0. [DOI] [PubMed] [Google Scholar]
- Newton A., Ohta N. Regulation of the cell division cycle and differentiation in bacteria. Annu Rev Microbiol. 1990;44:689–719. doi: 10.1146/annurev.mi.44.100190.003353. [DOI] [PubMed] [Google Scholar]
- Norris S. J., Charon N. W., Cook R. G., Fuentes M. D., Limberger R. J. Antigenic relatedness and N-terminal sequence homology define two classes of periplasmic flagellar proteins of Treponema pallidum subsp. pallidum and Treponema phagedenis. J Bacteriol. 1988 Sep;170(9):4072–4082. doi: 10.1128/jb.170.9.4072-4082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E. J., Bennett P. M. Structure of straight flagella from a mutant Salmonella. J Mol Biol. 1972 Sep 14;70(1):133–152. doi: 10.1016/0022-2836(72)90168-4. [DOI] [PubMed] [Google Scholar]
- Schmitt R., Raska I., Mayer F. Plain and complex flagella of Pseudomonas rhodos: analysis of fine structure and composition. J Bacteriol. 1974 Feb;117(2):844–857. doi: 10.1128/jb.117.2.844-857.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakihara Y., Wakabayashi T. Three-dimensional image reconstruction of straight flagella from a mutant Salmonella typhimurium. J Mol Biol. 1979 Jul 5;131(3):485–507. doi: 10.1016/0022-2836(79)90004-4. [DOI] [PubMed] [Google Scholar]
- Sjoblad R. D., Emala C. W., Doetsch R. N. Invited review: bacterial flagellar sheaths: structures in search of a function. Cell Motil. 1983;3(1):93–103. doi: 10.1002/cm.970030108. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J. A molecular switch: subunit rotations involved in the right-handed to left-handed transitions of Salmonella typhimurium flagellar filaments. J Mol Biol. 1991 Jul 5;220(1):67–77. doi: 10.1016/0022-2836(91)90381-f. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J., Aizawa S., Macnab R. M. Pairwise perturbation of flagellin subunits. The structural basis for the differences between plain and complex bacterial flagellar filaments. J Mol Biol. 1986 Aug 20;190(4):569–576. doi: 10.1016/0022-2836(86)90242-1. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J., Macnab R. M. Three-dimensional structure of the complex flagellar filament of Rhizobium lupini and its relation to the structure of the plain filament. J Mol Biol. 1987 Jun 5;195(3):603–620. doi: 10.1016/0022-2836(87)90185-9. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J. Three-dimensional reconstruction of the flagellar filament of Caulobacter crescentus. A flagellin lacking the outer domain and its amino acid sequence lacking an internal segment. J Mol Biol. 1988 Aug 20;202(4):787–808. doi: 10.1016/0022-2836(88)90559-1. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., DeRosier D. J. Three-dimensional structure of the frozen-hydrated flagellar filament. The left-handed filament of Salmonella typhimurium. J Mol Biol. 1987 Jun 5;195(3):581–601. doi: 10.1016/0022-2836(87)90184-7. [DOI] [PubMed] [Google Scholar]
- Trachtenberg S., Hammel I. The rigidity of bacterial flagellar filaments and its relation to filament polymorphism. J Struct Biol. 1992 Jul-Aug;109(1):18–27. doi: 10.1016/1047-8477(92)90063-g. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., DeRosier D. J., Aizawa S., Macnab R. M. Flagellar hook structures of Caulobacter and Salmonella and their relationship to filament structure. J Mol Biol. 1982 Nov 25;162(1):69–87. doi: 10.1016/0022-2836(82)90162-0. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., DeRosier D., Shapiro L., Weissborn A. Three-dimensional reconstruction of the flagellar hook from Caulobacter crescentus. J Mol Biol. 1981 Sep 25;151(3):439–465. doi: 10.1016/0022-2836(81)90005-x. [DOI] [PubMed] [Google Scholar]
- Wall J. S., Hainfeld J. F. Mass mapping with the scanning transmission electron microscope. Annu Rev Biophys Biophys Chem. 1986;15:355–376. doi: 10.1146/annurev.bb.15.060186.002035. [DOI] [PubMed] [Google Scholar]
- Weissborn A., Steinmann H. M., Shapiro L. Characterization of the proteins of the Caulobacter crescentus flagellar filament. Peptide analysis and filament organization. J Biol Chem. 1982 Feb 25;257(4):2066–2074. [PubMed] [Google Scholar]
- Yang G. C., Schrank G. D., Freeman B. A. Purification of flagellar cores of Vibrio cholerae. J Bacteriol. 1977 Feb;129(2):1121–1128. doi: 10.1128/jb.129.2.1121-1128.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]