Abstract
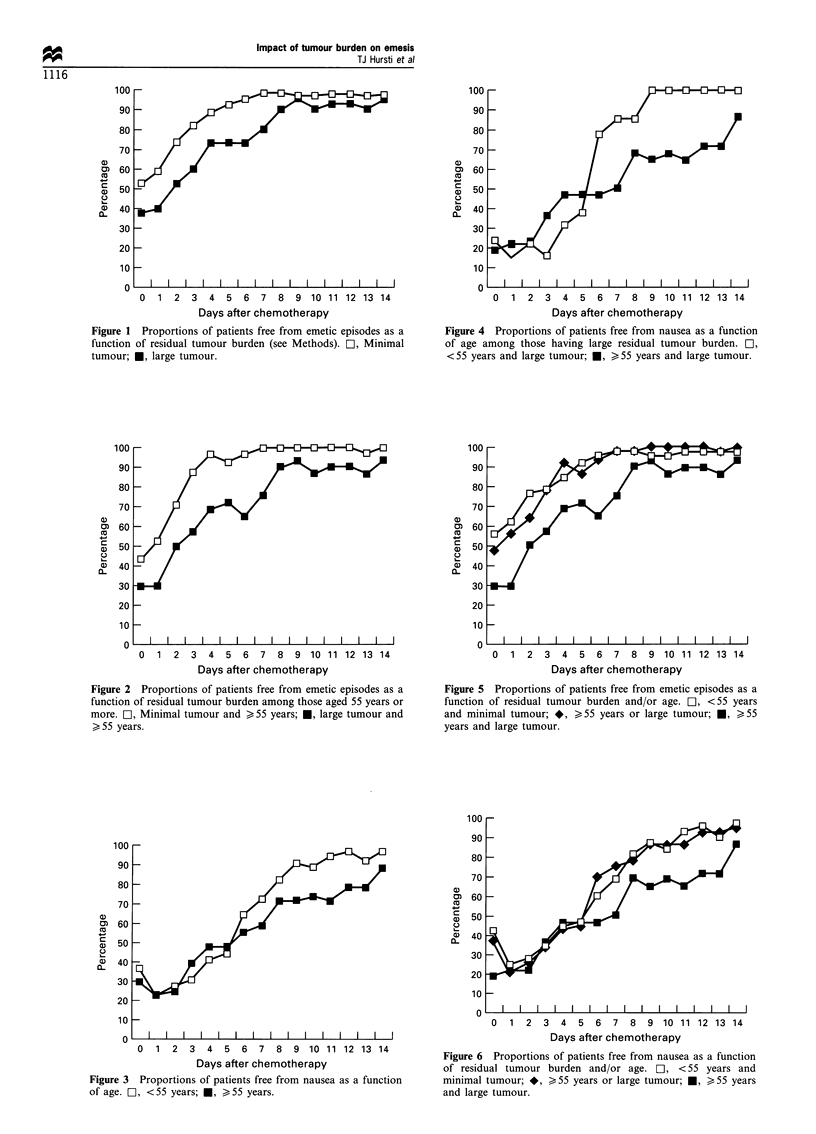
We investigated how residual tumour burden after cytoreductive surgery was related to the occurrence of acute and delayed nausea and vomiting in 101 ovarian cancer patients receiving their first chemotherapy course. The anti-emetic treatment included ondansetron combined with dexamethasone or placebo. After chemotherapy all patients received ondansetron only for 5 days. Two categories of tumour burden (TB) were formed according to the diameter of the greatest residual tumour (< 2 cm = minimal TB and > or = 2 cm = large TB). Self-reports of nausea and vomiting were obtained for 15 days. Other potential predictor variables were assessed and included in multivariate analyses. Patients with large compared with minimal TB had more delayed emesis, especially on days 2-7. They also had more acute nausea. The aggravating effect associated with large residual TB was more evident in patients > or = 55 years. During the second week after the chemotherapy the occurrence of nausea was higher in patients > or = 55 years than in those < 55 years. This was seen primarily in patients with large residual TB. Predictors for no delayed emesis at all were anti-emetic treatment with dexamethasone, minimal tumour burden, low neuroticism and no history of motion sickness. The increased risk of "persistent' delayed nausea and vomiting seen in older patients with large tumour burden may have important clinical implications and warrants further attention.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts D. S., Dahlberg S., Green S. J., Garcia D., Hannigan E. V., O'Toole R., Stock-Novack D., Surwit E. A., Malviya V. K., Jolles C. J. Analysis of patient age as an independent prognostic factor for survival in a phase III study of cisplatin-cyclophosphamide versus carboplatin-cyclophosphamide in stages III (suboptimal) and IV ovarian cancer. A Southwest Oncology Group study. Cancer. 1993 Jan 15;71(2 Suppl):618–627. doi: 10.1002/cncr.2820710220. [DOI] [PubMed] [Google Scholar]
- Andrykowski M. A., Gregg M. E. The role of psychological variables in post-chemotherapy nausea: anxiety and expectation. Psychosom Med. 1992 Jan-Feb;54(1):48–58. doi: 10.1097/00006842-199201000-00007. [DOI] [PubMed] [Google Scholar]
- Artinian N. T., Duggan C., Miller P. Age differences in patient recovery patterns following coronary artery bypass surgery. Am J Crit Care. 1993 Nov;2(6):453–461. [PubMed] [Google Scholar]
- Carmichael J., Bessell E. M., Harris A. L., Hutcheon A. W., Dawes P. J., Daniels S., Bessel E. M. Comparison of granisetron alone and granisetron plus dexamethasone in the prophylaxis of cytotoxic-induced emesis. Br J Cancer. 1994 Dec;70(6):1161–1164. doi: 10.1038/bjc.1994.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler W. B., Balducci L. Treatment considerations for older patients with cancer. In Vivo. 1994 Nov-Dec;8(5):737–744. [PubMed] [Google Scholar]
- Fredrikson M., Hursti T. J., Steineck G., Fürst C. J., Börjesson S., Peterson C. Delayed chemotherapy-induced nausea is augmented by high levels of endogenous noradrenaline. Br J Cancer. 1994 Oct;70(4):642–645. doi: 10.1038/bjc.1994.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrikson M., Hursti T., Fürst C. J., Steineck G., Börjeson S., Wikblom M., Peterson C. Nausea in cancer chemotherapy is inversely related to urinary cortisol excretion. Br J Cancer. 1992 May;65(5):779–780. doi: 10.1038/bjc.1992.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst C. J., Johansson S., Fredrikson M., Hursti T., Steineck G., Peterson C. Control of cisplatin induced emesis--a multidisciplinary intervention strategy. Med Oncol Tumor Pharmacother. 1992;9(2):81–86. doi: 10.1007/BF02989658. [DOI] [PubMed] [Google Scholar]
- Gandara D. R., Harvey W. H., Monaghan G. G., Perez E. A., Hesketh P. J. Delayed emesis following high-dose cisplatin: a double-blind randomised comparative trial of ondansetron (GR 38032F) versus placebo. Eur J Cancer. 1993;29A Suppl 1:S35–S38. doi: 10.1016/s0959-8049(05)80259-x. [DOI] [PubMed] [Google Scholar]
- Greenland S., Robins J. M. Estimation of a common effect parameter from sparse follow-up data. Biometrics. 1985 Mar;41(1):55–68. [PubMed] [Google Scholar]
- Heron J. F., Goedhals L., Jordaan J. P., Cunningham J., Cedar E. Oral granisetron alone and in combination with dexamethasone: a double-blind randomized comparison against high-dose metoclopramide plus dexamethasone in prevention of cisplatin-induced emesis. The Granisetron Study Group. Ann Oncol. 1994 Sep;5(7):579–574. doi: 10.1093/oxfordjournals.annonc.a058927. [DOI] [PubMed] [Google Scholar]
- Hursti T. J., Fredrikson M., Steineck G., Börjeson S., Fürst C. J., Peterson C. Endogenous cortisol exerts antiemetic effect similar to that of exogenous corticosteroids. Br J Cancer. 1993 Jul;68(1):112–114. doi: 10.1038/bjc.1993.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joss R. A., Bacchi M., Buser K., Kirchner V., Neuenschwander H., Orth B., Aapro M. S., Thürlimann B. Ondansetron plus dexamethasone is superior to ondansetron alone in the prevention of emesis in chemotherapy-naive and previously treated patients. Swiss Group for Clinical Cancer Research (SAKK). Ann Oncol. 1994 Mar;5(3):253–258. doi: 10.1093/oxfordjournals.annonc.a058803. [DOI] [PubMed] [Google Scholar]
- Kaizer L., Warr D., Hoskins P., Latreille J., Lofters W., Yau J., Palmer M., Zee B., Levy M., Pater J. Effect of schedule and maintenance on the antiemetic efficacy of ondansetron combined with dexamethasone in acute and delayed nausea and emesis in patients receiving moderately emetogenic chemotherapy: a phase III trial by the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994 May;12(5):1050–1057. doi: 10.1200/JCO.1994.12.5.1050. [DOI] [PubMed] [Google Scholar]
- Lindley C. M., Bernard S., Fields S. M. Incidence and duration of chemotherapy-induced nausea and vomiting in the outpatient oncology population. J Clin Oncol. 1989 Aug;7(8):1142–1149. doi: 10.1200/JCO.1989.7.8.1142. [DOI] [PubMed] [Google Scholar]
- Martin M., Diaz-Rubio E. Emesis during past pregnancy: a new prognostic factor in chemotherapy-induced emesis. Ann Oncol. 1990;1(2):152–153. doi: 10.1093/oxfordjournals.annonc.a057695. [DOI] [PubMed] [Google Scholar]
- Morrow G. R. The effect of a susceptibility to motion sickness on the side effects of cancer chemotherapy. Cancer. 1985 Jun 15;55(12):2766–2770. doi: 10.1002/1097-0142(19850615)55:12<2766::aid-cncr2820551207>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- O'Brien B. J., Rusthoven J., Rocchi A., Latreille J., Fine S., Vandenberg T., Laberge F. Impact of chemotherapy-associated nausea and vomiting on patients' functional status and on costs: survey of five Canadian centres. CMAJ. 1993 Aug 1;149(3):296–302. [PMC free article] [PubMed] [Google Scholar]
- Pennings J. L., Bachulis B. L., Simons C. T., Slazinski T. Survival after severe brain injury in the aged. Arch Surg. 1993 Jul;128(7):787–794. doi: 10.1001/archsurg.1993.01420190083011. [DOI] [PubMed] [Google Scholar]
- Roila F., Boschetti E., Tonato M., Basurto C., Bracarda S., Picciafuoco M., Patoia L., Santi E., Penza O., Ballatori E. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991 Jun;14(3):238–242. doi: 10.1097/00000421-199106000-00010. [DOI] [PubMed] [Google Scholar]
- Ruff P., Paska W., Goedhals L., Pouillart P., Rivière A., Vorobiof D., Bloch B., Jones A., Martin C., Brunet R. Ondansetron compared with granisetron in the prophylaxis of cisplatin-induced acute emesis: a multicentre double-blind, randomised, parallel-group study. The Ondansetron and Granisetron Emesis Study Group. Oncology. 1994 Jan-Feb;51(1):113–118. doi: 10.1159/000227321. [DOI] [PubMed] [Google Scholar]
- Sorbe B., Högberg T., Himmelmann A., Schmidt M., Räisänen I., Stockmeyer M., de Bruijn K. M. Efficacy and tolerability of tropisetron in comparison with a combination of tropisetron and dexamethasone in the control of nausea and vomiting induced by cisplatin-containing chemotherapy. Eur J Cancer. 1994;30A(5):629–634. doi: 10.1016/0959-8049(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Tonato M., Roila F., Del Favero A. Methodology of antiemetic trials: a review. Ann Oncol. 1991 Feb;2(2):107–114. doi: 10.1093/oxfordjournals.annonc.a057871. [DOI] [PubMed] [Google Scholar]
- de Wet M., Falkson G., Rapoport B. L. Repeated use of granisetron in patients receiving cytostatic agents. Cancer. 1993 Jun 15;71(12):4043–4049. doi: 10.1002/1097-0142(19930615)71:12<4043::aid-cncr2820711239>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- du Bois A., Meerpohl H. G., Vach W., Kommoss F. G., Fenzl E., Pfleiderer A. Course, patterns, and risk-factors for chemotherapy-induced emesis in cisplatin-pretreated patients: a study with ondansetron. Eur J Cancer. 1992;28(2-3):450–457. doi: 10.1016/s0959-8049(05)80075-9. [DOI] [PubMed] [Google Scholar]


