Abstract
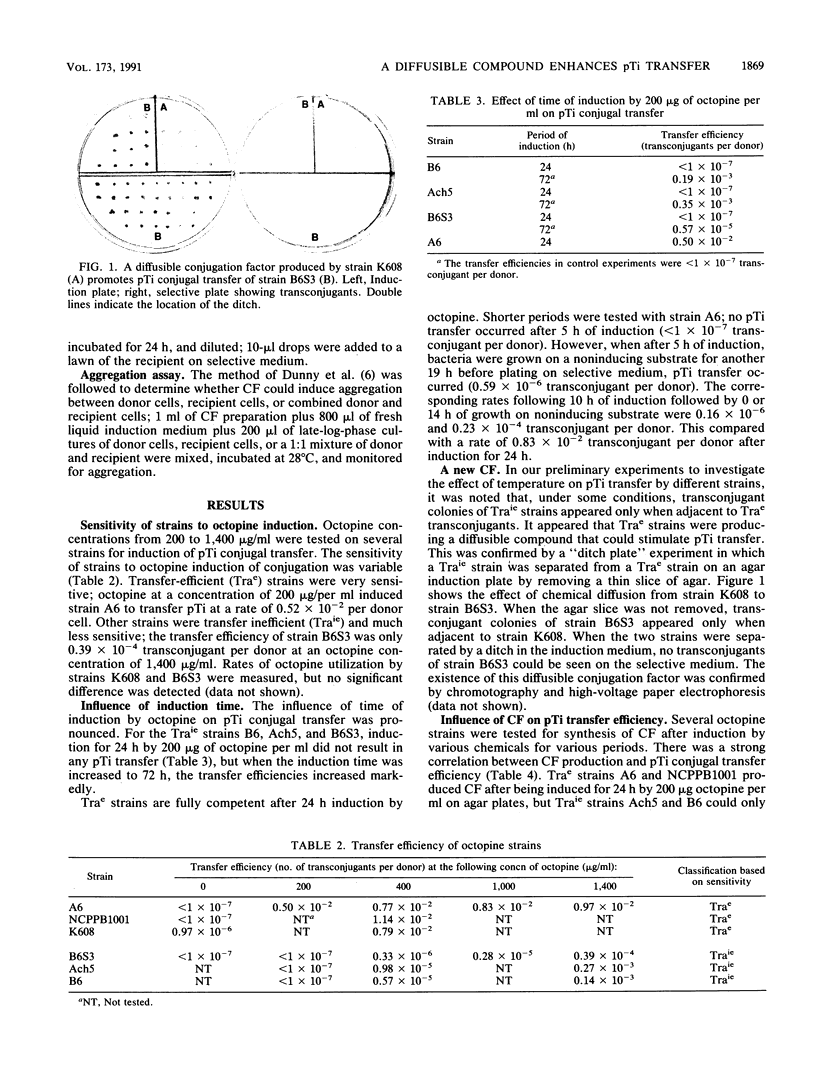
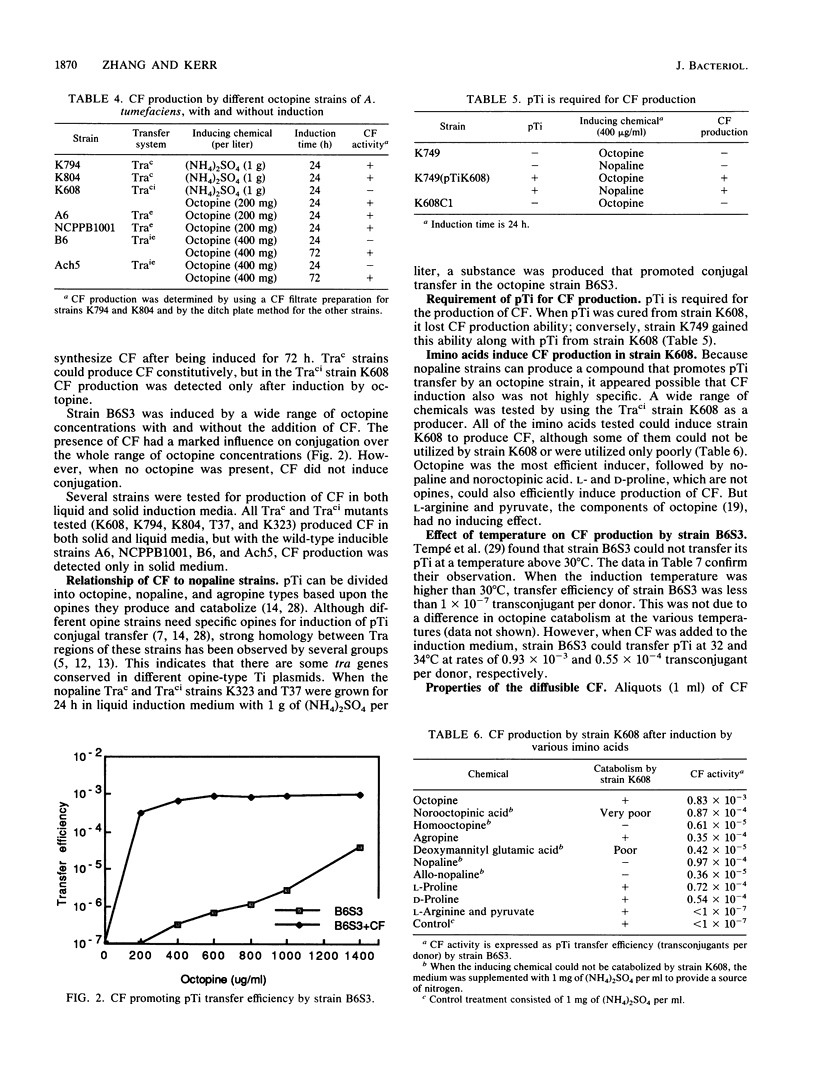
Several octopine strains of Agrobacterium tumefaciens were tested for Ti plasmid (pTi) transfer after induction by 400 micrograms of octopine per ml for 24 h. The strains could be divided into two groups, transfer efficient (Trae) and transfer inefficient (Traie); the respective rates of transfer were 0.77 x 10(-2) to 1.14 x 10(-2) and 0.33 x 10(-6) to 9.8 x 10(-6) plasmid transconjugant per donor cell. Transfer efficiencies of Traie strains were greatly increased when the time of induction was 72 h. A diffusible conjugation factor (CF) that can enhance conjugal transfer of pTi in A. tumefaciens was discovered when both Trae and Traie donor strains were induced in the same plate. The evidence indicates that CF is a key factor affecting transfer efficiency of pTi but is not sufficient by itself to induce transfer. Trac mutants can produce CF constitutively, and Trae strains can produce it after induction by low octopine concentrations. The transfer efficiency of Traie strains was greatly increased by adding CF to the induction medium. The thermosensitive strain B6S, which normally cannot conjugate at temperatures above 30 degrees C, could transfer pTi efficiently at 32 and 34 degrees C in the presence of CF. Production of CF is dependent on the presence of pTi but appears to be common for different opine strains; it was first detected in octopine strains, but nopaline strains also produced the same or a similar compound. CF is very biologically active, affecting donor but not recipient bacterial cells, but CF does not promote aggregation. Data suggest that CF might be an activator or derepressor in the conjugation system of A. tumefaciens. CF is a dialyzable small molecule and is resistant to DNase, RNase, protease, and heating to 100 degrees C for 10 min, but autoclaving (121 degrees C for 15 min) and alkaline treatment removed all activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clare B. G., Kerr A., Jones D. A. Characteristics of the nopaline catabolic plasmid in Agrobacterium strains K84 and K1026 used for biological control of crown gall disease. Plasmid. 1990 Mar;23(2):126–137. doi: 10.1016/0147-619x(90)90031-7. [DOI] [PubMed] [Google Scholar]
- De Greve H., Decraemer H., Seurinck J., Van Montagu M., Schell J. The functional organization of the octopine Agrobacterium tumefaciens plasmid pTiB6s3. Plasmid. 1981 Sep;6(2):235–248. doi: 10.1016/0147-619x(81)90069-x. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. G., Kerr A., Tempé J., Petit A. Arginine catabolism: a new function of both octopine and nopaline Ti-plasmids of Agrobacterium. Mol Gen Genet. 1979 Jun 20;173(3):263–269. doi: 10.1007/BF00268636. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B., Habeck L. L. vir genes influence conjugal transfer of the Ti plasmid of Agrobacterium tumefaciens. J Bacteriol. 1990 Mar;172(3):1600–1608. doi: 10.1128/jb.172.3.1600-1608.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetello C., Van Larebeke N., Holsters M., De Picker A., Van Montagu M., Schell J. Ti plasmids of Agrobacterium as conjugative plasmids. Nature. 1977 Feb 10;265(5594):561–563. doi: 10.1038/265561a0. [DOI] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Kerr A., Manigault P., Tempé J. Transfer of virulence in vivo and in vitro in Agrobacterium. Nature. 1977 Feb 10;265(5594):560–561. doi: 10.1038/265560a0. [DOI] [PubMed] [Google Scholar]
- Klapwijk P. M., Scheulderman T., Schilperoort R. A. Coordinated regulation of octopine degradation and conjugative transfer of Ti plasmids in Agrobacterium tumefaciens: evidence for a common regulatory gene and separate operons. J Bacteriol. 1978 Nov;136(2):775–785. doi: 10.1128/jb.136.2.775-785.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapwijk P. M., Schilperoort R. A. Negative control of octopine degradation and transfer genes of octopine Ti plasmids in Agrobacterium tumefaciens. J Bacteriol. 1979 Aug;139(2):424–431. doi: 10.1128/jb.139.2.424-431.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott J. A., Beiderbeck R., Lippincott B. B. Utilization of octopine and nopaline by Agrobacterium. J Bacteriol. 1973 Oct;116(1):378–383. doi: 10.1128/jb.116.1.378-383.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENAGE A., MOREL G. SUR LA PR'ESENCE D'OCTOPINE DANS LES TISSUS DE CROWN-GALL. C R Hebd Seances Acad Sci. 1964 Dec 21;259:4795–4796. [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. Agrobacterium tumefaciens and the susceptible plant cell: a novel adaptation of extracellular recognition and DNA conjugation. Cell. 1986 Oct 24;47(2):155–157. doi: 10.1016/0092-8674(86)90437-x. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Kado C. I. Virulence genes promote conjugative transfer of the Ti plasmid between Agrobacterium strains. J Bacteriol. 1990 Apr;172(4):2191–2193. doi: 10.1128/jb.172.4.2191-2193.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempé J., Petit A., Holsters M., Montagu M., Schell J. Thermosensitive step associated with transfer of the Ti plasmid during conjugation: Possible relation to transformation in crown gall. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Krishnan M., Gould J. H., Smith R. H., Gelvin S. B. Opines stimulate induction of the vir genes of the Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1989 Jul;171(7):3696–3703. doi: 10.1128/jb.171.7.3696-3703.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet G., Holsters M., Teuchy H., Van Montagu M., Schell J. Characterization of different plaque-forming and defective temperate phages in Agrobacterium. J Gen Virol. 1975 Jan;26(1):33–48. doi: 10.1099/0022-1317-26-1-33. [DOI] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P. Basic processes underlying Agrobacterium-mediated DNA transfer to plant cells. Annu Rev Genet. 1988;22:1–30. doi: 10.1146/annurev.ge.22.120188.000245. [DOI] [PubMed] [Google Scholar]
- Zambryski P., Tempe J., Schell J. Transfer and function of T-DNA genes from agrobacterium Ti and Ri plasmids in plants. Cell. 1989 Jan 27;56(2):193–201. doi: 10.1016/0092-8674(89)90892-1. [DOI] [PubMed] [Google Scholar]
- von Bodman S. B., McCutchan J. E., Farrand S. K. Characterization of conjugal transfer functions of Agrobacterium tumefaciens Ti plasmid pTiC58. J Bacteriol. 1989 Oct;171(10):5281–5289. doi: 10.1128/jb.171.10.5281-5289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]



