Abstract
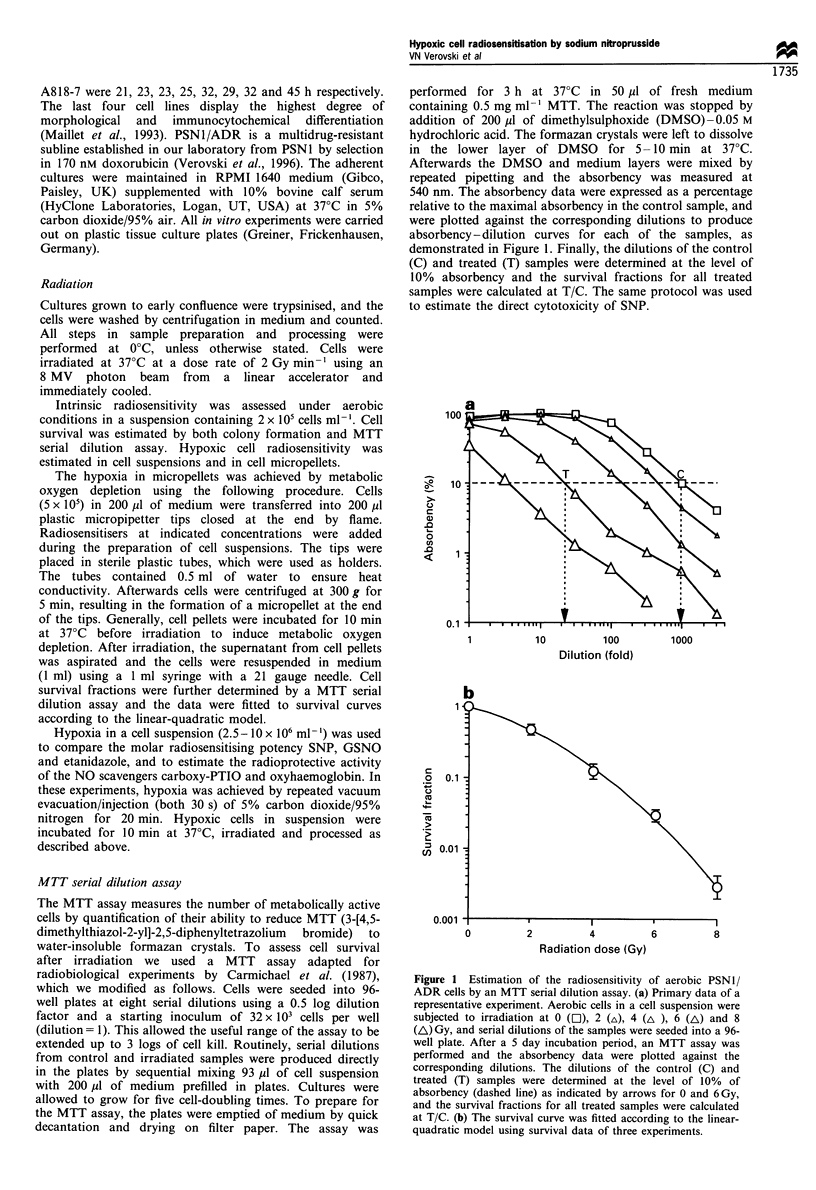
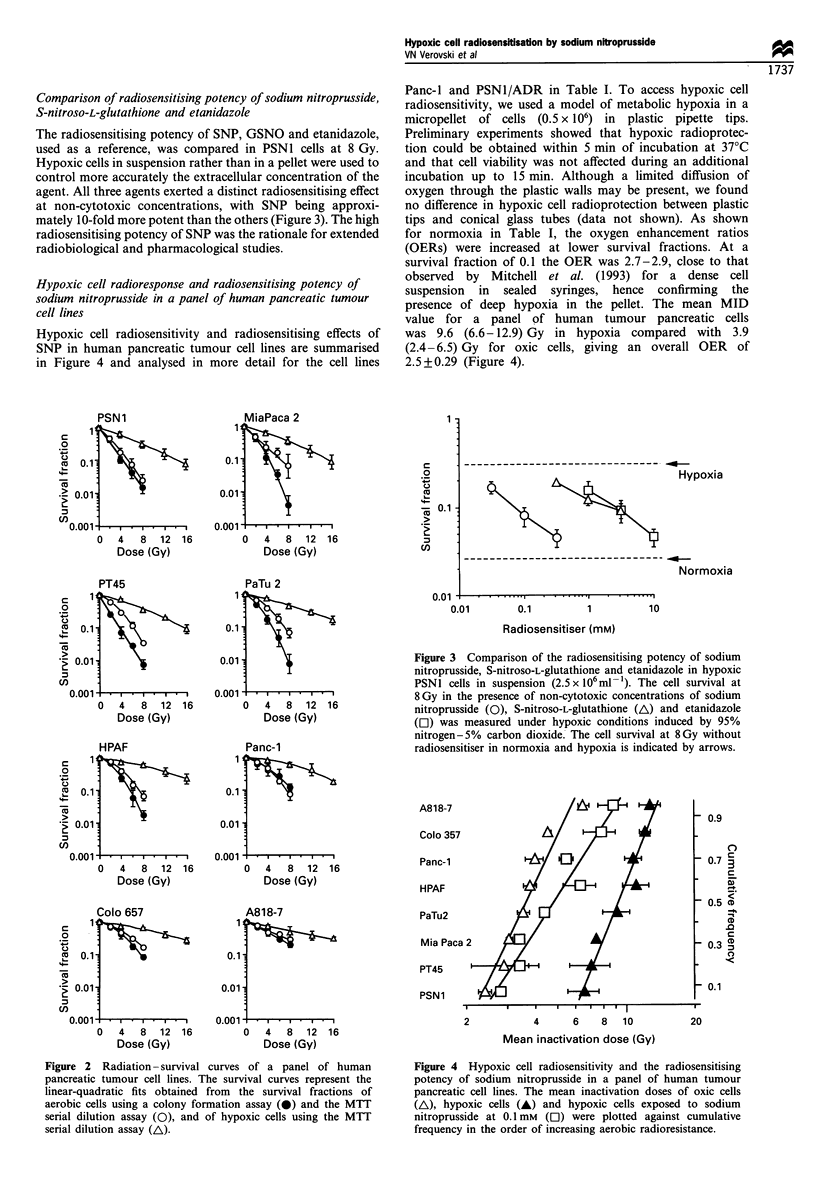
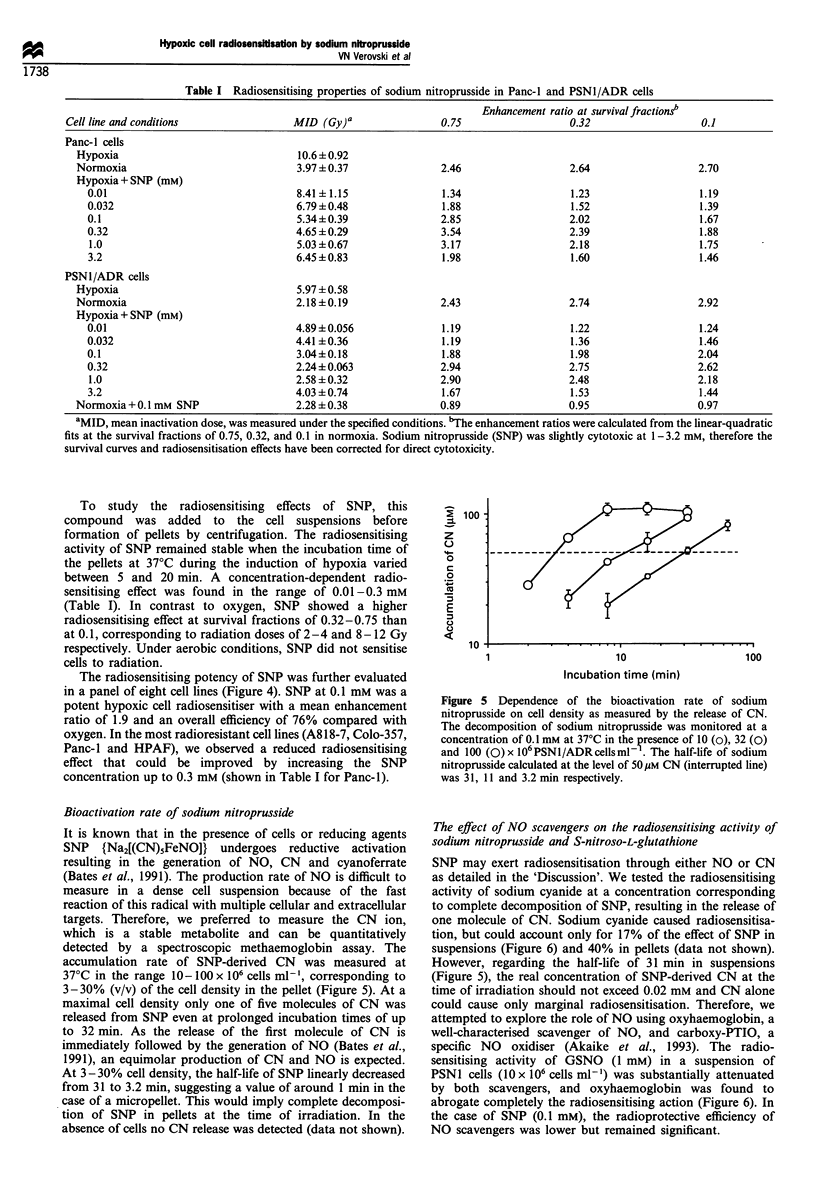
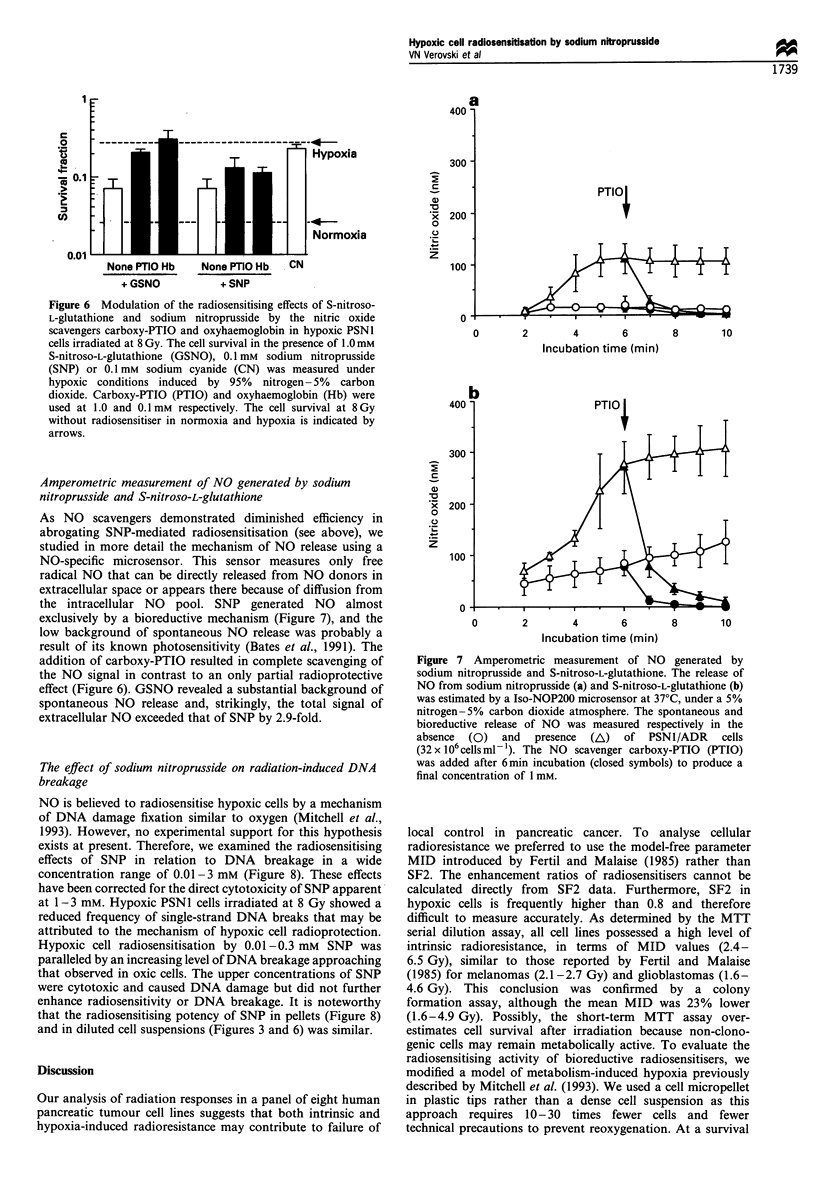
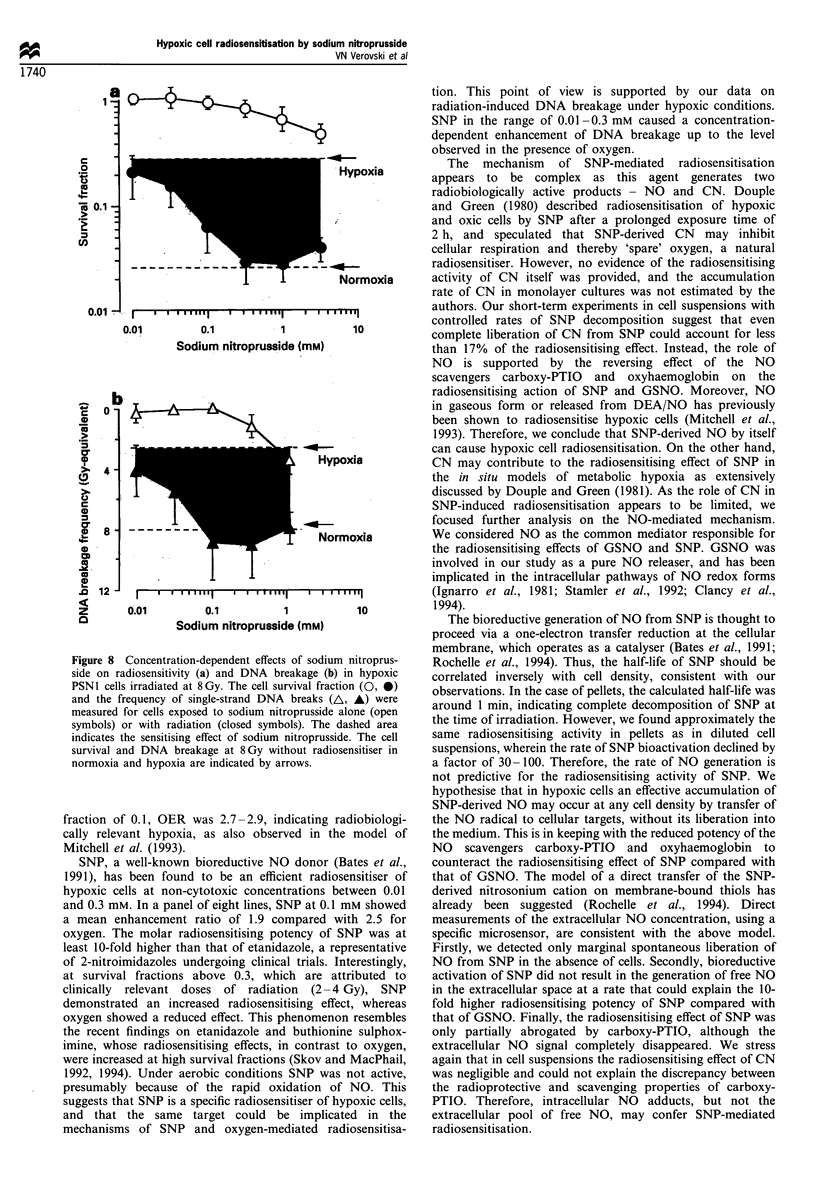
A panel of eight human pancreatic tumour cell lines displayed high intrinsic radioresistance, with mean inactivation doses between 2.4 and 6.5 Gy, similar to those reported for melanoma and glioblastoma. The radiosensitising potency of sodium nitroprusside, a bioreductive nitric oxide donor, was assessed in a model of metabolism-induced hypoxia in a cell micropellet. Sodium nitroprusside at 0.1 mM revealed a radiosensitising effect with an overall enhancement ratio of 1.9 compared with 2.5 for oxygen. Radiosensitising activity correlated with the enhancement of single-strand DNA breakage caused by radiation. In suspensions with cell densities of between 3% and 30% (v/v), the half-life of sodium nitroprusside decreased from 31 to 3.2 min, suggesting a value of around 1 min for micropellets. Despite this variation, the radiosensitising activity was similar in micropellets and in diluted cell suspensions. S-nitroso-L-glutathione was found to possess radiosensitising activity, consistent with a possible role of natural thiols in the storing of radiobiologically active nitric oxide adducts derived from sodium nitroprusside. As measured by a nitric oxide-specific microsensor, activation of sodium nitroprusside occurred by bioreduction, whereas S-nitroso-L-glutathione showed substantial spontaneous decomposition. Both agents appear to exert radiosensitising action through nitric oxide as its scavenging by carboxy phenyltetramethylimidazolineoxyl N-oxide (carboxy-PTI0) and oxyhaemoglobin resulted in attenuated radiosensitisation. Sodium nitroprusside was at least 10-fold more potent than etanidazole, a 2-nitroimidazole used as a reference. Our data suggest that sodium nitroprusside, a drug currently used for the treatment of hypertension, is a potential tumour radioresponse modifier.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E. Failla Memorial Lecture. Redox, radiation, and reductive bioactivation. Radiat Res. 1992 Nov;132(2):129–139. [PubMed] [Google Scholar]
- Akaike T., Yoshida M., Miyamoto Y., Sato K., Kohno M., Sasamoto K., Miyazaki K., Ueda S., Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993 Jan 26;32(3):827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- Bates J. N., Baker M. T., Guerra R., Jr, Harrison D. G. Nitric oxide generation from nitroprusside by vascular tissue. Evidence that reduction of the nitroprusside anion and cyanide loss are required. Biochem Pharmacol. 1991 Dec 11;42 (Suppl):S157–S165. doi: 10.1016/0006-2952(91)90406-u. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987 Feb 15;47(4):943–946. [PubMed] [Google Scholar]
- Clancy R. M., Levartovsky D., Leszczynska-Piziak J., Yegudin J., Abramson S. B. Nitric oxide reacts with intracellular glutathione and activates the hexose monophosphate shunt in human neutrophils: evidence for S-nitrosoglutathione as a bioactive intermediary. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3680–3684. doi: 10.1073/pnas.91.9.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvaeye M., Verovski V., De Neve W., Storme G. DNA breakage, cytotoxicity, drug accumulation and retention in two human ovarian tumor cell lines AZ224 and AZ364 treated with adriamycin, modulated by verapamil. Anticancer Res. 1993 Sep-Oct;13(5A):1533–1538. [PubMed] [Google Scholar]
- Douple E. B., Green C. J., Simic M. G. Potentiation of cellular radiosensitivity by nitroprusside and vitamin B12. Int J Radiat Oncol Biol Phys. 1980 Nov;6(11):1545–1549. doi: 10.1016/0360-3016(80)90013-9. [DOI] [PubMed] [Google Scholar]
- Fertil B., Malaise E. P. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys. 1985 Sep;11(9):1699–1707. doi: 10.1016/0360-3016(85)90223-8. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P. Effect of nitric oxide on the radiosensitivity of bacteria. Nature. 1957 Nov 30;180(4596):1191–1192. doi: 10.1038/1801191a0. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994 Mar 1;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos C. M., Morley D., Wink D. A., Dunams T. M., Saavedra J. E., Hoffman A., Bove A. A., Isaac L., Hrabie J. A., Keefer L. K. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J Med Chem. 1991 Nov;34(11):3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- Maragos C. M., Wang J. M., Hrabie J. A., Oppenheim J. J., Keefer L. K. Nitric oxide/nucleophile complexes inhibit the in vitro proliferation of A375 melanoma cells via nitric oxide release. Cancer Res. 1993 Feb 1;53(3):564–568. [PubMed] [Google Scholar]
- Mitchell J. B., Wink D. A., DeGraff W., Gamson J., Keefer L. K., Krishna M. C. Hypoxic mammalian cell radiosensitization by nitric oxide. Cancer Res. 1993 Dec 15;53(24):5845–5848. [PubMed] [Google Scholar]
- Rochelle L. G., Kruszyna H., Kruszyna R., Barchowsky A., Wilcox D. E., Smith R. P. Bioactivation of nitroprusside by porcine endothelial cells. Toxicol Appl Pharmacol. 1994 Sep;128(1):123–128. doi: 10.1006/taap.1994.1189. [DOI] [PubMed] [Google Scholar]
- Skov K. A., MacPhail H. S. Effect of BSO on the radiation response at low (0-4 Gy) doses. Int J Radiat Oncol Biol Phys. 1992;22(3):533–536. doi: 10.1016/0360-3016(92)90869-j. [DOI] [PubMed] [Google Scholar]
- Skov K. A., MacPhail S. Low concentrations of nitroimidazoles: effective radiosensitizers at low doses. Int J Radiat Oncol Biol Phys. 1994 Apr 30;29(1):87–93. doi: 10.1016/0360-3016(94)90230-5. [DOI] [PubMed] [Google Scholar]
- Smith R. P., Kruszyna H. Nitroprusside produces cyanide poisoning via reaction with hemoglobin. J Pharmacol Exp Ther. 1974 Dec;191(3):557–563. [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Holden S. A., Northey D., Dewhirst M. W., Herman T. S. Therapeutic effect of infused Fluosol-DA/carbogen with ephedrine, flunarizine, or nitroprusside. Int J Radiat Oncol Biol Phys. 1993 Apr 30;26(1):103–109. doi: 10.1016/0360-3016(93)90179-y. [DOI] [PubMed] [Google Scholar]
- Verovski V. N., Van den Berge D. L., Delvaeye M. M., Scheper R. J., De Neve W. J., Storme G. A. Low-level doxorubicin resistance in P-glycoprotein-negative human pancreatic tumour PSN1/ADR cells implicates a brefeldin A-sensitive mechanism of drug extrusion. Br J Cancer. 1996 Mar;73(5):596–602. doi: 10.1038/bjc.1996.103. [DOI] [PMC free article] [PubMed] [Google Scholar]


