Abstract
Hypothalamic fatty acid metabolism has recently been implicated in the controls of food intake and energy homeostasis. We report that intracerebroventricular (ICV) injection of leptin, concomitant with inhibiting AMP-activated kinase (AMPK), activates acetyl-CoA carboxylase (ACC), the key regulatory enzyme in fatty acid biosynthesis, in the arcuate nucleus (Arc) and paraventricular nucleus (PVN) in the hypothalamus. Arc overexpression of constitutively active AMPK prevents the Arc ACC activation in response to ICV leptin, supporting the hypothesis that AMPK lies upstream of ACC in leptin's Arc intracellular signaling pathway. Inhibiting hypothalamic ACC with 5-tetradecyloxy-2-furoic acid, a specific ACC inhibitor, blocks leptin-mediated decreases in food intake, body weight, and mRNA level of the orexigenic neuropeptide NPY. These results show that hypothalamic ACC activation makes an important contribution to leptin's anorectic effects. Furthermore, we find that ICV leptin up-regulates the level of malonyl-CoA (the intermediate of fatty acid biosynthesis) specifically in the Arc and increases the level of palmitoyl-CoA (a major product of fatty acid biosynthesis) specifically in the PVN. The rises of both levels are blocked by 5-tetradecyloxy-2-furoic acid along with the blockade of leptin-mediated hypophagia. These data suggest malonyl-CoA as a downstream mediator of ACC in leptin's signaling pathway in the Arc and imply that palmitoyl-CoA, instead of malonyl-CoA, could be an effector in relaying ACC signaling in the PVN. Together, these findings highlight site-specific impacts of hypothalamic ACC activation in leptin's anorectic signaling cascade.
Keywords: carnitine palmitoyltransferase, long-chain fatty acyl CoA, malonyl CoA, oleic acid, malonyl CoA decarboxylase
Energy balance is maintained by hypothalamic systems responding to hormonal and neural signals that sense body energy status (1). Leptin is an anorexigenic hormone secreted mainly from adipocytes that controls food intake and energy homeostasis primarily by acting at hypothalamic nuclei such as the arcuate nucleus (Arc) (1, 2). The Arc, the primary nucleus in the hypothalamus in mediating leptin's control of energy balance, contains first-order neurons that express leptin receptors and a variety of feeding-related neuropeptides such as neuropeptide Y (NPY), agouti-related peptide (AgRP), and α-melanocyte stimulating hormone (α-MSH) (1). Leptin exerts its anorectic effects by modulating the levels of these neuropeptides. Activation of signal transducer and activator of transcription 3 (STAT3) and activation of phosphatidylinositol 3-kinase have been shown to play critical roles in leptin's hypothalamic intracellular signaling pathways (3, 4).
Further aspects of leptin's hypothalamic intracellular signaling have been identified. Minokoshi et al. (5) have demonstrated that exogenous administration of leptin inhibits AMP-activated kinase (AMPK) in the Arc, and, based on the finding that constitutive activity of AMPK in the Arc prevents leptin's anorectic actions, they suggest that the Arc AMPK inhibition is necessary for leptin's anorectic signaling (5). One well characterized substrate of AMPK is acetyl-CoA carboxylase (ACC), and AMPK inactivates ACC by phosphorylating its serine residue (Ser-79) (6, 7). ACC is the key regulatory enzyme of the biosynthesis of fatty acids such as palmitate, and it catalyzes the formation of malonyl-CoA, the intermediate in the fatty acid synthetic pathway and a regulator of mitochondrial long-chain fatty acyl-CoA uptake (8, 9). We predicted that leptin-mediated AMPK inhibition would activate ACC (by preventing Ser phosphorylation), which, in turn, would increase metabolic flux in the fatty acid synthetic pathway. We expected that the level of malonyl-CoA, the product of ACC, and/or palmitate, a direct product of the pathway, would be elevated. Both metabolites have been suggested to play significant roles in the hypothalamic controls of food intake and energy balance (8, 10–15). In this report, we provide evidence supporting the hypothesis that ACC plays an important role in the hypothalamic intracellular signaling cascade underlying leptin's controls of food intake and energy balance. Our data also highlight site-specific potential roles for the metabolites of the fatty acid biosynthetic pathway in leptin's hypothalamic regulation of energy homeostasis.
Results
Intracerebroventricular (ICV) Leptin Activates ACC Concomitant with Inhibition of AMPK in the Arc and Paraventricular Nucleus (PVN).
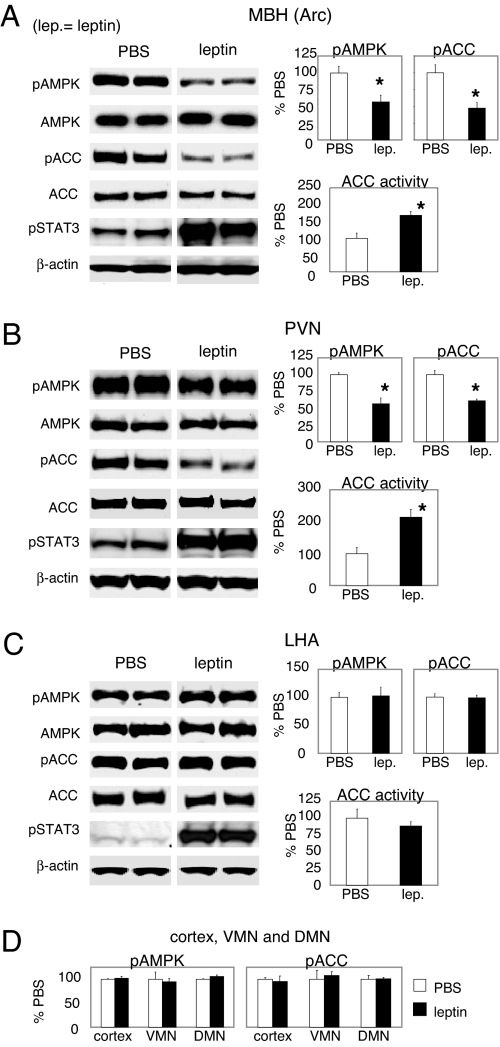
The Arc, located in the mediobasal hypothalamus (MBH), contains leptin-responsive neurons. The paraventricular nucleus (PVN) and lateral hypothalamic area (LHA), two projection sites from the Arc, contain second-order neurons that mediate leptin's anorectic actions (1). We examined the activities of AMPK and ACC in these nuclei in response to ICV administration of leptin. Activation of AMPK usually requires Thr-172 phosphorylation in its α catalytic subunit, and the level of phosphorylation correlates well with activity (16–18). ICV leptin decreased Arc pAMPK, indicating AMPK inhibition, and decreased phospho (Ser-79)-ACC (pACC), indicating ACC activation (Fig. 1A). As expected, leptin increased pSTAT3 level in the Arc (Fig. 1A). The activation of ACC by leptin in the Arc was confirmed by using an ACC activity assay (Fig. 1A). ICV leptin also inhibited AMPK in the PVN, as indicated by a decreased level of PVN pAMPK (Fig. 1B). Concomitant with the inhibition of AMPK, pACC was reduced, and ACC activity was increased in the PVN after ICV leptin (Fig. 1B). The decreases in pACC levels in both Arc and PVN appeared to be selective to the α isoform of ACC because the phosphorylation levels of ACC β-isoform were not significantly affected by leptin [supporting information (SI) Fig. 5]. In the LHA, leptin increased pSTAT3 level (Fig. 1C) but did not affect the level of pAMPK (Fig. 1C), pACC-α (Fig. 1C), or pACC-β (data not shown). ACC activity in the LHA was not affected by leptin, consistent with the lack of change in the pACC level (Fig. 1C). There were no changes of pAMPK or pACC in ventromedial nucleus (VMN), dorsomedial nucleus (DMN) or cortex (Fig. 1D). Thus, our data show that ICV leptin induces the activation of hypothalamic ACC specifically in the Arc and the PVN.
Fig. 1.
ICV leptin activates hypothalamic ACC in both Arc and PVN. Rats were fasted overnight before receiving ICV injection of leptin. The rats were euthanized at 3 h after ICV injection. Western blotting and ACC activity assay were performed to analyze the responses of AMPK and ACC to ICV leptin. (A) Levels of pAMPK and pACC (n = 13–16) and ACC activity (n = 7) in the Arc. (B) Levels of pAMPK and pACC (n = 14) and ACC activity (n = 7) in the PVN. (C) Levels of pAMPK and pACC (n = 8–14) and ACC activity (n = 7) in the LHA. (D) Levels of pAMPK and pACC in the cortex, ventromedial nucleus (VMN), and dorsomedial nucleus (DMN) (n = 4–5). *, P < 0.05.
Arc Overexpression of Constitutively Active AMPK Blocks Leptin-Mediated ACC Activation in both Arc and PVN.
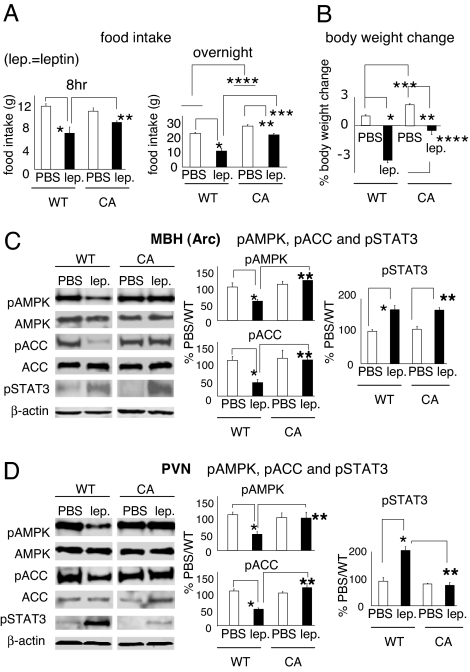
To determine whether AMPK inhibition in the Arc is required for ACC activation in response to leptin administration, we expressed constitutively active AMPK (CA-AMPK) in the mediobasal hypothalamus (mainly the Arc) using a recombinant adenovirus. Constitutive activity of AMPK was verified in cultured A549 cells by monitoring the phosphorylation level (Ser-79) of ACC (SI Fig. 6A). In rats injected with the virus, CA-AMPK expression was confined to the MBH (mainly the Arc), as evidenced by the expression pattern of hemagglutinin tag of CA-AMPK (SI Fig. 6B). Arc overexpression of CA-AMPK induced orexigenic effects starting on day 3 after virus injection (SI Fig. 6 C and D and Fig. 2 A and B), consistent with the data from a prior study in mice using the same adenovirus (5). We then administered leptin ICV to rats injected with the adenovirus and monitored their food intake and body weight. Arc overexpression of CA-AMPK reduced the leptin-mediated inhibition of food intake and loss of body weight (Fig. 2 A and B). In support of the constitutive activity of AMPK in the Arc, leptin failed to inhibit Arc AMPK in CA-AMPK rats (Fig. 2C). Importantly, the ACC activation in the Arc after ICV leptin was fully blocked in the rats with Arc overexpression of CA-AMPK (Fig. 2C), supporting the hypothesis that AMPK inhibition is necessary for ACC activation in leptin's Arc signaling cascade. Consistent with the previous finding (5), Arc overexpression of CA-AMPK did not affect leptin's ability to increase pSTAT3 in the Arc (Fig. 2C), suggesting that Arc AMPK signaling is downstream of, or independent of, the STAT3 pathway in the Arc (5). Arc overexpression of CA-AMPK blocked the ability of ICV leptin to inhibit AMPK, activate ACC, and increase pSTAT3 level in the PVN (Fig. 2D), suggesting that AMPK inhibition by leptin in the Arc is necessary for leptin's effects on AMPK, ACC, and STAT3 in the PVN. This finding is consistent with other recent data suggesting indirect actions of leptin in the PVN. For example, although the PVN contains leptin receptors (1), leptin-induced activation of PVN neurons has been demonstrated to be mediated through melanocortin receptors located in the PVN that respond to the neuropeptides (such as AgRP and α-MSH) originated from the Arc (19). In our experiment, the constitutive activity of AMPK was confined to the MBH(Arc) (SI Fig. 6B), the impaired PVN signaling in the CA-AMPK animals in response to leptin thus indicated a necessary contribution from AMPK inhibition in the Arc to the PVN intracellular pathways in leptin's hypothalamic signaling cascade. It should be noted that Arc overexpression of CA-AMPK did not increase the basal (WT/PBS) level of pAMPK in the Arc (Fig. 2C), which is consistent with the finding and interpretation of Minokoshi et al. (5). In both studies, the animals had been fasted before they were given leptin. Because fasting itself elevated the levels of pAMPK and pACC in the Arc (data not shown), a fasted state may mask the already elevated levels of pAMPK and/or pACC in the Arc in CA-AMPK rats.
Fig. 2.
ICV leptin fails to reduce pACC level in the Arc and the PVN in CA-AMPK rats. (A and B) Recombinant adenoviruses expressing H150R γ1 subunit of AMPK (CA-AMPK, tagged with HA) or wild-type γ1 subunit of AMPK (WT-AMPK) were injected into the mediobasal hypothalamus (MBH, mainly Arc) of rats. Four days after virus injection, leptin was administered at 2 h before dark onset. Food intakes, 8 h and overnight after ICV leptin (A) and overnight body weight changes (B) were monitored. Food intake/body weight change from each rat having a targeted MBH expression of HA tag was included (CA-AMPK: n = 10). WT-AMPK rats were used as control (n = 11). (A) *, P < 0.001; **, P ≤ 0.05; ***, P < 0.005; ****, (PBS/WT vs. PBS/CA), P < 0.01. (B) *, P < 0.001; **, P < 0.005; ***, P < 0.005; ****, P < 0.001. (C and D) Seven days after virus injection, the rats were fasted overnight and were given an ICV injection of leptin the next day (i.e., the 8th day after virus injection). They were euthanized at 3 h after leptin injection. The levels of pAMPK and pACC in the Arc (C, n = 18–21) and the PVN (D, n = 16) were quantitated by Western blotting. The pSTAT3 levels in the Arc (C, n = 9) and the PVN (D, n = 8) were quantitated. The rat having a targeted MBH expression of HA tag was used in the Western blotting analysis. * and **, P < 0.05.
Hypothalamic ACC Activation Is Important for Leptin's Anorectic Actions.
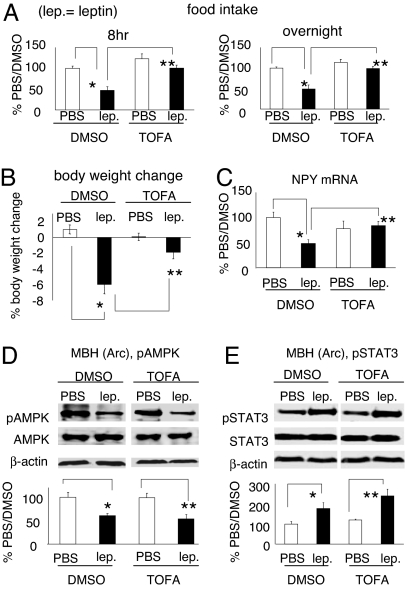
To assess the role of hypothalamic ACC in mediating the anorectic actions of exogenous leptin, we administered leptin to rats treated with an ACC inhibitor. Because ACC exists in two isoforms (α and β) in the hypothalamus (20, 21), the effect of disrupting one isoform might be offset by a potential compensatory activation of the other. We therefore ICV infused rats with 5-tetradecyloxy-2-furoic acid (TOFA), a specific and dual inhibitor of ACC (12, 22, 23). In our studies, ICV infusion of TOFA reduced hypothalamic malonyl-CoA levels, indicating ACC inhibition (SI Fig. 7C). Consistent with prior reports (10, 12, 14, 24), central infusion of TOFA had no significant effect on the basal (PBS/DMSO) level of food intake (Fig. 3A) or body weight (Fig. 3B), thus ruling out the possibility of an independent orexigenic effect from TOFA at this dose (14). Importantly, we found that TOFA abolished the ability of ICV leptin to inhibit food intake and it attenuated the body weight loss by leptin (Fig. 3 A and B). TOFA's ability to block the leptin-mediated anorectic actions is unlikely to be due to a nonspecific action on the hypothalamus because we were able to inhibit food intake with the anorectic compound melanotan-II (25) in rats receiving central TOFA infusion (a 50% reduction of overnight food intake by melanotan-II in both DMSO-infused rats and TOFA-infused rats). We also examined the role of ACC activation in leptin-mediated hypothalamic neuropeptide signaling. A single ICV bolus injection of leptin down-regulated the mRNA level of the orexigenic neuropeptide NPY in rats (21 h after leptin), and TOFA blocked this down-regulation (Fig. 3C) (Note: mRNA levels of POMC and AgRP were not altered by leptin administration or TOFA infusion in this paradigm). These results indicate that hypothalamic ACC activation is important for leptin's anorectic effects. TOFA did not interfere with the ability of leptin to decrease pAMPK level or to increase pSTAT3 level in the Arc (Fig. 3 D and E), arguing against the view that TOFA blocks leptin's anorectic actions by impairing leptin-induced AMPK or STAT3 signaling in the Arc. These data also imply that ACC functions either downstream of, or in a parallel pathway to, STAT3 in leptin's Arc signaling pathway.
Fig. 3.
Inhibition of hypothalamic ACC by TOFA blocks leptin's anorectic actions without interfering with the response of AMPK or STAT3 in the Arc to ICV leptin. Rats were infused with TOFA or DMSO into their lateral ventricle for 2–3 days for habituation. On day 3 to day 5 after infusion, rats were given ICV leptin at 2 h before dark onset. (A and B) ICV TOFA infusion prevented leptin-induced food intake inhibition (8 h and overnight after ICV leptin) and body weight loss (24 h), n = 7–8 per group. Eight-hour food intake: *, P < 0.005; **, P < 0.001; overnight food intake: *, P < 0.005; **, P ≤ 0.001; body weight change: *, P < 0.001; **, P < 0.005. (C) ICV TOFA infusion blocked the down-regulation of NPY mRNA level by leptin. NPY mRNA levels (21 h after ICV bolus injection) were measured by real-time quantitative PCR (n = 4–5 per group). mRNA of cyclophilin was used as the loading control. *, P < 0.005; **, P < 0.05. (D and E) ICV TOFA infusion did not prevent the leptin-mediated decrease in the pAMPK level or increase in the pSTAT3 level in the Arc (n = 3–5 per group). * and **, P < 0.05.
ICV Leptin Increases Malonyl-CoA Level in the Arc and Increases Palmitoyl-CoA Level in the PVN.
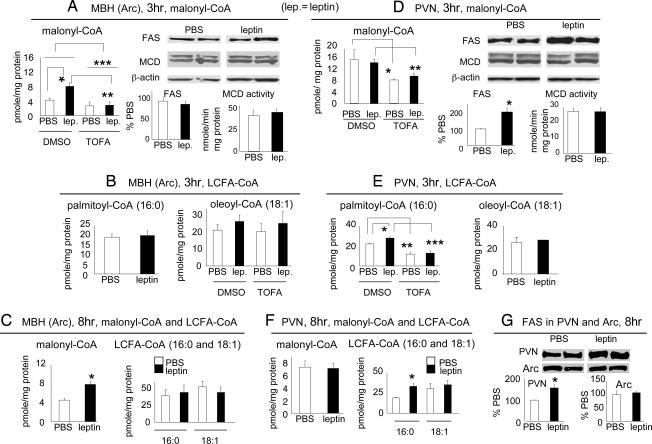
To evaluate the potential downstream mediators through which leptin activation of hypothalamic ACC affects food intake, we quantified malonyl-CoA and long-chain fatty acyl-CoA (LCFA-CoA) levels in individual hypothalamic nuclei in response to ICV leptin. Prior work quantifying malonyl-CoA level from whole hypothalamic lysate has suggested a role for malonyl-CoA as a satiety factor (12, 14). Consistent with such a role, we demonstrated that ICV leptin increased malonyl-CoA level in the Arc, the primary region in the hypothalamus in the regulation of energy balance, and TOFA prevented this increase (Fig. 4A). Leptin did not affect blood glucose level (SI Fig. 7D), ruling out a potential contribution of blood glucose to the changes in Arc malonyl-CoA level. Malonyl-CoA is synthesized by carboxylation of acetyl-CoA by ACC and degraded by malonyl-CoA decarboxylase (MCD) (26), and malonyl-CoA is the substrate for fatty acid synthase (FAS) in fatty acid biosynthesis (9). To explore the mechanism for the up-regulation of malonyl-CoA level by leptin, we evaluated whether ICV leptin would produce changes in FAS or MCD. FAS and MCD protein levels in the Arc were not affected by ICV leptin (Fig. 4A). Because FAS level correlates well with activity (14, 20), our data suggest that leptin does not affect FAS activity in the Arc. Leptin also did not alter MCD activity in the Arc (Fig. 4A). Hence, ICV leptin appears to increase the malonyl-CoA level secondary to increasing ACC activity. Malonyl-CoA is a known physiological inhibitor of carnitine palmitoyltransferase-1 (CPT-1), the enzyme facilitating mitochondrial uptake of LCFA for β-oxidation (8). As a result, the increased malonyl-CoA in the Arc in response to leptin would be expected to increase the level of LCFA-CoA through inhibiting CPT-1. Because LCFA (or LCFA-CoA), notably oleic acid, has been implicated in the hypothalamic control of food intake (11), we determined the levels of two major LCFA-CoAs, i.e., palmitoyl-CoA (16:0) and oleoyl-CoA (18:1), after ICV leptin. In the Arc, there were no significant effects of leptin on the levels of these LCFA-CoAs 3 h after treatment (Fig. 4B), a time point at which the malonyl-CoA level in the Arc was significantly elevated. There was a notable trend of increase in the level of oleoyl-CoA after leptin, but the trend was not affected by TOFA treatment (Fig. 4B), suggesting that the trend was not secondary to the leptin-mediated up-regulation of ACC activity in the Arc and the consequent increase in the level of Arc malonyl-CoA. Consistent with a dissociation between malonyl-CoA and LCFA-CoA in mediating leptin's anorectic effects, at 8 h, when leptin inhibition on food intake became obvious, Arc malonyl-CoA level remained increased, however, the trend for the increase in oleoyl-CoA diminished (Fig. 4C).
Fig. 4.
ICV leptin increases malonyl-CoA level in the Arc and increases palmitoyl-CoA level in the PVN. (A and D) TOFA- or DMSO-infused rats received an ICV injection of leptin on day 5 after infusion. Rats were euthanized 3 h after ICV injection, and malonyl-CoA levels in the Arc (A, n = 20) and the PVN (D, two PVN extracts were pooled for use in one reaction, n = 11) were quantified. (A) *, P < 0.01; **, P = 0.001; ***, P < 0.005. (D) * and **, P < 0.05. Protein levels of FAS (Arc, n = 7; PVN, n = 12) and MCD (n = 5–7) were analyzed by Western blotting (D). *, P < 0.05. MCD activity assay was performed in the Arc and the PVN, n = 7 for each nucleus. Note: the basal (PBS/DMSO) level of malonyl-CoA in the PVN is three to four times greater than that in the Arc. This difference in the basal levels may explain why TOFA decreased the PVN, but not the Arc, malonyl-CoA level. (B and E) TOFA- or DMSO-infused rats were euthanized at 3 h after ICV leptin. Palmitoyl-CoA (16:0) and oleoyl-CoA (18:1) levels in the Arc (B, n = 21) and the PVN (E, n = 11) were quantified. (E) *, P < 0.05; **, P < 0.005; ***, P < 0.001. (C and F) Rats received an ICV injection of leptin and were euthanized 8 h later. The levels of malonyl-CoA and LCFA-CoA (palmitoyl-CoA and oleoyl-CoA) in the Arc (C, n = 7) and the PVN (F, n = 7) were quantified. *, P < 0.05. (G) FAS protein levels (8 h after leptin) in the Arc and the PVN were analyzed by Western blotting, n = 7 (PVN: *, P < 0.05).
In the PVN, at 3 h after leptin, malonyl-CoA level did not increase (Fig. 4D) despite the activation of ACC (Fig. 1B). ICV leptin increased the FAS level (Fig. 4D) suggesting FAS activation, which would enhance incorporation of malonyl-CoA into palmitate (and palmitoyl-CoA). As in the Arc, central leptin did not affect MCD activity in the PVN (Fig. 4D). We found a significant increase of palmitoyl-CoA (16:0) level (but no change in malonyl-CoA level) in the PVN 3 h after leptin (Fig. 4E), which could be caused by increased ACC activity together with FAS up-regulation. TOFA infusion decreased the levels of both malonyl-CoA (Fig. 4D) and palmitoyl-CoA (16:0) (Fig. 4E) in the PVN, and leptin did not increase the palmitoyl-CoA level in TOFA-infused rats (Fig. 4E). These results suggest that leptin-mediated ACC activation is required for the increase of palmitoyl-CoA level in the PVN. At 8 h after leptin, we found a similar pattern of changes in the PVN, i.e., AMPK was inhibited (data not shown), ACC was activated (data not shown), malonyl-CoA level was not affected (Fig. 4F), and FAS protein level (Fig. 4G) and palmitoyl-CoA (16:0) level (Fig. 4F) remained elevated. The elevation in LCFA-CoA level appears to be specific to palmitoyl-CoA. At both 3 h and 8 h after leptin, the level of oleoyl-CoA (18:1), a major downstream product from palmitoyl-CoA in de novo synthesis of LCFA-CoA (27), did not change (Fig. 4 E and F). The synthesis of oleoyl-CoA requires stearoyl-CoA desaturase (27), which might not be affected by leptin treatment. Finally, consistent with the absence of leptin-induced alteration in LHA ACC (Fig. 1C), there was no change in the malonyl-CoA level in the LHA after ICV leptin (SI Fig. 7E).
Discussion
Our results demonstrate that central administration of leptin activates ACC in both Arc and PVN, two major hypothalamic sites in the controls of food intake and energy homeostasis. The activation of ACC appears to be important for leptin-mediated anorectic actions and inhibition of orexigenic neuropeptide gene expression, because blockade of hypothalamic ACC with TOFA prevented the full expression of these actions after central leptin. Because ACC is the key regulatory enzyme of biosynthesis of fatty acids such as palmitate, activation of ACC is expected to elevate the level of malonyl-CoA (the intermediate) and/or palmitate (and palmitoyl-CoA) (a direct de novo product). Indeed, we found that ICV leptin specifically increases malonyl-CoA level in the Arc and palmitoyl-CoA level in the PVN. The leptin-mediated increase in Arc malonyl-CoA is prevented by TOFA treatment as is the leptin's anorectic effects. In the Arc, constitutive activation of AMPK abolishes leptin's ability to activate ACC, indicating that AMPK inhibition is required for ACC activation in leptin's Arc signaling cascade. Together, our results support the hypothesis that ACC links AMPK and malonyl-CoA in the Arc to mediate leptin's anorectic actions. In the PVN, in addition to ACC activation, FAS protein level was up-regulated after ICV leptin. The up-regulation of FAS would enhance the incorporation of malonyl-CoA into palmitoyl-CoA and would account for the absence of increase in malonyl-CoA level in the presence of the ACC activation. Importantly, TOFA treatment that blocked leptin inhibition of food intake also prevented the leptin-mediated increase in palmitoyl-CoA level in the PVN. Thus, these results leave open the possibility for palmitoyl-CoA in relaying the signal from ACC in leptin's PVN intracellular pathway.
Unlike the change in the Arc malonyl-CoA level, the change in the Arc LCFA-CoA level was not consistent with the leptin-induced alteration in food intake. Both malonyl-CoA and LCFA-CoA such as oleoyl-CoA have been implicated in the hypothalamic control of food intake (8, 10–15), and a specific role for the Arc malonyl-CoA in the hypothalamic actions of leptin has been proposed (15). Our data do not offer positive evidence for a necessary link between malonyl-CoA and LCFA-CoA in the Arc in mediating leptin's anorectic signaling. In our studies, ICV leptin did not significantly affect the LCFA-CoA levels in the Arc. Although the trend for the increase of the Arc LCFA-CoA (oleoyl-CoA) level after leptin may well imply a potentially meaningful rise of LCFA-CoA in a specific subset of neurons and/or a specific cellular fraction (e.g., cytosol) in the Arc, such a trend was not affected by TOFA inhibition on ACC. The small increase is unlikely to be caused by the increased malonyl-CoA (resulting from ACC activation) and a consequent potential inhibition of CPT-1, because TOFA treatment that blocks the elevation of Arc malonyl-CoA level fails to prevent the small increase. Our data thus imply that there could be mechanisms for up-regulating LCFA-CoA level other than the malonyl-CoA-mediated CPT-1 inhibition. In support of such a prediction, we found that ICV leptin induced a significant and substantial elevation of palmitoyl-CoA and oleoyl-CoA level in the LHA even though the ACC activity and the malonyl-CoA level were not altered by leptin at this site (SI Fig. 7 E and F). Together, our data suggest that LCFA-CoA and malonyl-CoA could function in nuclei-specific ways to mediate leptin's anorectic signaling cascade.
The mechanism through which elevated malonyl-CoA may mediate the signal from ACC activation in the Arc to reduce food intake has yet to be determined. The liver isoform of CPT1 (CPT-1A) is much more abundant than the muscle isoform (CPT-1B) in the Arc (8), and CPT-1A has a fairly low binding affinity for malonyl-CoA (28). As a result, the leptin-induced increase in the malonyl-CoA level might not have been sufficient for inhibiting CPT-1A in the Arc. Alternatively, malonyl-CoA could relay the signal from ACC by interacting with the recently identified brain isoform of CPT-1 (CPT-1C) that does not use LCFA-CoA as substrate but binds malonyl-CoA (29). Malonyl-CoA could also affect food intake independent of CPT-1.
In summary, our data provide evidence for hypothalamic ACC activation as an important contributor to leptin's anorectic actions. The effects of leptin-induced ACC activation appear to be nuclei specific. In the Arc, the primary hypothalamic site mediating leptin's controls of food intake and energy balance, our results support the hypothesis that ACC links AMPK and malonyl-CoA in mediating leptin's intracellular signaling cascade. In the PVN, a secondary hypothalamic nucleus in mediating the actions of leptin, our data are consistent with a potential role for palmitate (or palmitoyl-CoA) in mediating leptin's anorectic signaling. A diagram of hypothalamic fatty acid biosynthetic pathway in mediating leptin intracellular signaling is shown in SI Fig. 8. Together, these data suggest that modulation of hypothalamic ACC activity is a promising strategy for maintaining body energy balance or treating obesity.
Materials and Methods
Animals.
Animal experiments were done in accordance with the guidelines on animal care and use established by the Johns Hopkins University School of Medicine Institutional Animal Care and Use Committee. Male Sprague–Dawley rats (225–275 g) were purchased from the Charles River Laboratories (Wilmington, MA), housed in a controlled (12-h light/12-h dark) environment, and allowed ad lib access to standard laboratory chow and water unless otherwise noted.
Cannulation Surgery.
Rats were implanted with cannulas into either lateral or 3rd ventricle. The coordinates for lateral ventricular injection were: anterior–posterior (from bregma): −1.0 mm; dorsal–ventral (from skull surface): −6.2 mm; and medial–lateral: 1.4 mm. The coordinates for third ventricular cannulation were: anterior–posterior (from lamda): +4.6–5.7 mm; dorsal–ventral (from skull surface): −8.5 mm; and medial–lateral: midline of the skull. Cannula placements were verified by assessing a drinking response to angiotensin II or a feeding response to NPY.
Central Infusion of TOFA by Osmotic Minipump.
The pilot data showed that bolus ICV injection of DMSO (a vehicle necessary for dissolving TOFA) was not well tolerated by rats. We then chose to deliver TOFA (a generous gift from Craig A. Townsend, Department of Chemistry at The Johns Hopkins University) by slow central infusion based on previous studies (24, 30). TOFA (15 μg/0.5 μl/hr) or DMSO was infused via osmotic minipump (DURECT) into lateral ventricle (LV) of rats (30). Similar LV minipump infusion procedures demonstrated that TOFA blocked the anorectic actions by central administration of glucose and insulin (24). The chronically infused rats were implanted with cannulas into the other lateral ventricle or 3rd ventricle for bolus injection of leptin. Placement of the infusion cannula was verified by histological examination. LV infusion of DMSO did not significantly change daily food consumption or body weight gain from the basal (PBS infusion) level (SI Fig. 9 A and B).
ICV Injection of Leptin.
Leptin was obtained from A. F. Parlow, National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases. Dose–response study showed that a single bolus ICV injection of 15 μg of leptin dissolved in 1× PBS (vehicle) induced a consistent and robust reduction of food intake and body weight loss, and this dose of leptin also consistently and significantly decreased pAMPK level in the Arc and PVN. Therefore, we chose to ICV inject 15 μg of leptin in all our experiments.
Dissection of Hypothalamic Regions and Quantification of Hypothalamic Neuropeptides mRNA Level by Real-Time Quantitative PCR.
At a designated time point, rats were euthanized by decapitation. Brains were dissected within 1 min and immediately snap-frozen in isopentane on dry ice. Each hypothalamic region was quickly dissected from 600 μm of frozen coronal sections based on the coordinates described in ref. 31. Dissection of PVN and dorsomedial nucleus was verified by in situ hybridization experiment (data not shown). The accuracy of dissection of the other hypothalamic regions was validated by comparing the mRNA levels of characteristic neuropeptides in hypothalamic dissections by using quantitative RT-PCR (SI Fig. 10). The MBH included mainly the Arc.
Western Blotting.
Phospho-AMPK (Thr-172), AMPK α-subunit, phospho-STAT3 (Tyr-705), STAT3, and ACC-α isoform were determined by using corresponding antibodies from Cell Signaling Technology (Beverly, MA). Phospho-ACC was determined by using the antibody against phospho-Ser-79 of ACC α isoform from Upstate Biotechnology (Lake Placid, NY). FAS and β-actin were determined with antibodies from BD Biosciences (San Diego, CA) and Sigma (St. Louis, MO), respectively. HA antibody was from Roche (Indianapolis, IN). MCD antibody was generated and provided by Gary D. Lopaschuk at the University of Alberta. Densitometry was performed by using Scion Image software (Scion, Frederick, MD). The ratio of the intensity of the phospho-protein (e.g., pAMPK or pACC α-isoform) to that of the total protein (e.g., AMPK α-subunit or ACC α-isoform) was calculated to represent the level of phosphorylation. The phosphorylation level of ACC β-isoform was calculated as the ratio of the intensity of phospho-ACC-β (using the same Ab for detecting pACC-α) to that of β-actin (Note: the Ab against the ACC α-isoform failed to detect the ACC β-isoform). β-Actin was used as the loading control.
Activity Assay of ACC and MCD.
The procedure for ACC activity assay is described in SI Methods. MCD activity assay was performed based on the established protocol (26).
Quantification of Malonyl-CoA and LCFA-CoA.
Rats were fasted overnight before receiving ICV leptin. The levels of malonyl-CoA and LCFA-CoA (palmitoyl-CoA, 16:0; oleoyl-CoA, 18:1) in the MBH (Arc), PVN, and LHA were quantified. The procedures for malonyl-CoA recycling assay and LCFA-CoA quantification are described in SI Methods.
MBH Injection of Recombinant Adenovirus Expressing Constitutively Active AMPK.
Preliminary studies with dye injections were performed to target MBH for viral injections. Once the coordinates and injection volumes were determined, rats were injected with a recombinant adenovirus expressing H150R γ1 subunit (providing AMPK with constitutive activity) tagged with hemagglutinin or wild-type γ1 subunit of AMPK into mediobasal hypothalamus bilaterally (3 × 108 p.f.u./0.3 μl per side). The coordinates were: anterior–posterior (from bregma): −2.8 mm; dorsal–ventral (from dura): −9.0 mm; and medial–lateral: ±0.5 mm.
Statistical Analysis.
All values are presented as mean ± SEM. Effects of treatments were assessed by unpaired t test or ANOVA (individual differences among treatments were compared by Student–Newman–Keuls method).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK19302 and DK068054, the Paul R. McHugh Chair for Motivated Behavior, and a grant from the Canadian Institute of Health Research.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AMPK
AMP-activated kinase
- Arc
arcuate nucleus
- CA-AMPK
constitutively active AMPK
- CPT-1
carnitine palmitoyltransferase-1
- FAS
fatty acid synthase
- LCFA
long-chain fatty acid
- LHA
lateral hypothalamic area
- ICV
intracerebroventricular
- MBH
mediobasal hypothalamus
- MCD
malonyl-CoA decarboxylase
- NPY
neuropeptide Y
- PVN
paraventricular nucleus
- STAT3
signal transducer and activator of transcription 3
- TOFA
5-tetradecyloxy-2-furoic acid.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708385104/DC1.
References
- 1.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Schwartz MW. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 4.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 5.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, et al. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 6.Hardie DG. Prog Lipid Res. 1989;28:117–146. doi: 10.1016/0163-7827(89)90010-6. [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, Lopez-Casillas F, Bai DH, Luo X, Pape ME. FASEB J. 1989;3:2250–2256. doi: 10.1096/fasebj.3.11.2570725. [DOI] [PubMed] [Google Scholar]
- 8.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Nat Med. 2003;9:756–761. doi: 10.1038/nm873. [DOI] [PubMed] [Google Scholar]
- 9.Beaty NB, Lane MD. Ann NY Acad Sci. 1985;447:23–37. doi: 10.1111/j.1749-6632.1985.tb18423.x. [DOI] [PubMed] [Google Scholar]
- 10.Loftus TM, Jaworsky DE, Frehywot GL, Townsend CA, Ronnett GV, Lane MD, Kuhajda FP. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 11.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 12.Hu Z, Cha SH, Chohnan S, Lane MD. Proc Natl Acad Sci USA. 2003;100:12624–12629. doi: 10.1073/pnas.1834402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z, Dai Y, Prentki M, Chohnan S, Lane MD. J Biol Chem. 2005;280:39681–39683. doi: 10.1074/jbc.C500398200. [DOI] [PubMed] [Google Scholar]
- 14.Lopez M, Lelliott CJ, Tovar S, Kimber W, Gallego R, Virtue S, Blount M, Vazquez MJ, Finer N, Powles TJ, et al. Diabetes. 2006;55:1327–1336. doi: 10.2337/db05-1356. [DOI] [PubMed] [Google Scholar]
- 15.He W, Lam TK, Obici S, Rossetti L. Nat Neurosci. 2006;9:227–233. doi: 10.1038/nn1626. [DOI] [PubMed] [Google Scholar]
- 16.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 17.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. J Biol Chem. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 18.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 19.Zhang ZH, Felder RB. Am J Physiol. 2004;286:R303–R310. doi: 10.1152/ajpregu.00504.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kim EK, Miller I, Landree LE, Borisy-Rudin FF, Brown P, Tihan T, Townsend CA, Witters LA, Moran TH, Kuhajda FP, et al. Am J Physiol. 2002;283:E867–E879. doi: 10.1152/ajpendo.00178.2002. [DOI] [PubMed] [Google Scholar]
- 21.Gao S, Lane MD. Proc Natl Acad Sci USA. 2003;100:5628–5633. doi: 10.1073/pnas.1031698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCune SA, RA Harris. J Biol Chem. 1979;254:10095–10101. [PubMed] [Google Scholar]
- 23.Harada N, Oda Z, Hara Y, Fujinami K, Okawa M, Ohbuchi K, Yonemoto M, Ikeda Y, Ohwaki K, Aragane K, et al. Mol Cell Biol. 2007;27:1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert M, Magnan C, Turban S, Andre J, Guerre-Millo M. Diabetes. 2003;52:277–282. doi: 10.2337/diabetes.52.2.277. [DOI] [PubMed] [Google Scholar]
- 25.Kinzig KP, Scott KA, Hyun J, Bi S, Moran TH. Obes Res. 2005;13:1672–1682. doi: 10.1038/oby.2005.205. [DOI] [PubMed] [Google Scholar]
- 26.Dyck JRB, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD. Am J Physiol. 1998;275:H2122–H2129. doi: 10.1152/ajpheart.1998.275.6.H2122. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. J Biol Chem. 2000;275:30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 28.Esser V, Brown NF, Cowan AT, Foster DW, McGarry JD. J Biol Chem. 1996;271:6972–6977. doi: 10.1074/jbc.271.12.6972. [DOI] [PubMed] [Google Scholar]
- 29.Wolfgang MJ, Kurama T, Dai Y, Suwa A, Asaumi M, Matsumoto S, Cha SH, Shimokawa T, Lane MD. Proc Natl Acad Sci USA. 2006;103:7282–7287. doi: 10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahu A. J Neuroendocrinol. 2002;14:796–804. doi: 10.1046/j.1365-2826.2002.00840.x. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 5th Ed. New York: Academic; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.