Abstract
The Salmonella typhimurium prsB mutation was previously mapped at 45 min on the chromosome, and a prsB strain was reported to produce undetectable levels of phosphoribosylpyrophosphate (PRPP) synthetase activity and very low levels of immunologically cross-reactive protein in vitro (N.K. Pandey and R.L. Switzer, J. Gen. Microbiol, 128:1863-1871, 1982). We have shown by P22-mediated transduction that the prsB gene is actually an allele of prsA, the structural gene for PRPP synthetase, which maps at 35 min. The prsB (renamed prs-100) mutant produces about 20% of the activity and 100% of the cross-reactive material of wild-type strains. prs-100 mutant strains are temperature sensitive, as is the mutant PRPP synthetase in vitro. The prs-100 mutation is a C-to-T transition which results in replacement of Arg-78 in the mature wild-type enzyme by Cys. The mutant PRPP synthetase was purified to greater than 98% purity. It possessed elevated Michaelis constants for both ATP and ribose-5-phosphate, a reduced maximal velocity, and reduced sensitivity to the allosteric inhibitor ADP. The mutant enzyme had altered physical properties and was susceptible to specific cleavage at the Arg-101-to-Ser-102 bond in vivo. It appears that the mutation alters the enzyme's kinetic properties through substantial structural alterations rather than by specific perturbation of substrate binding or catalysis.
Full text
PDF



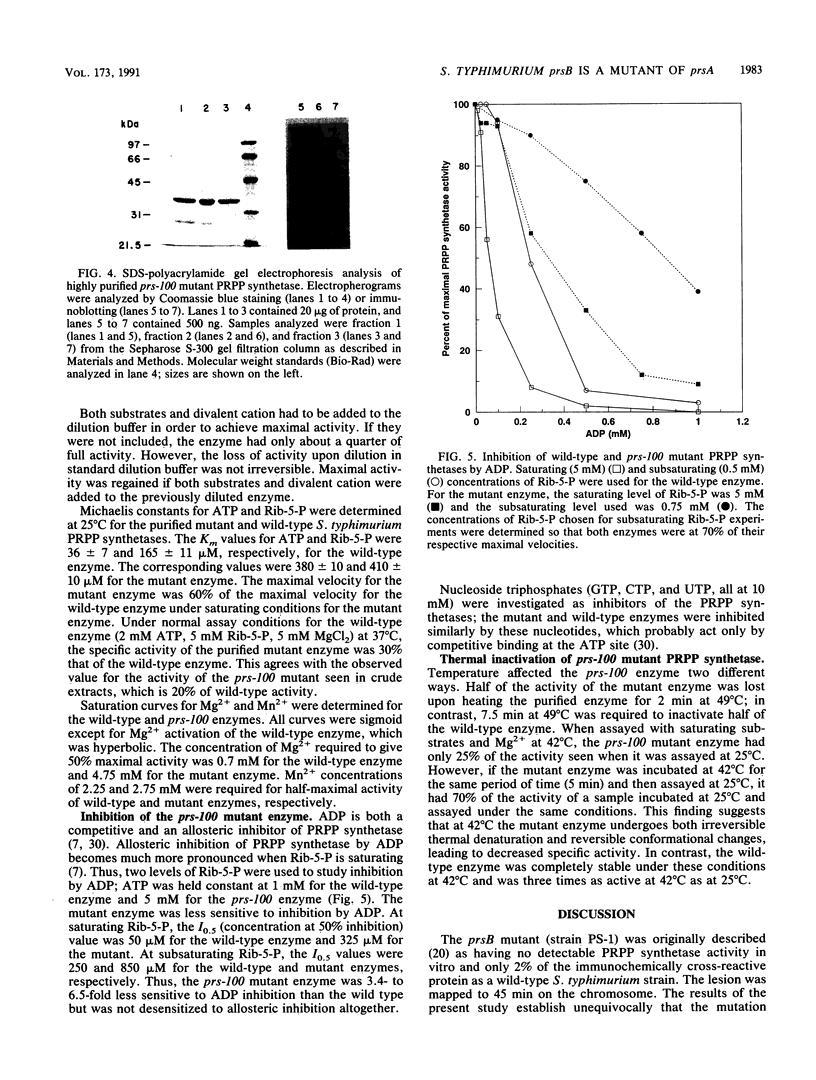



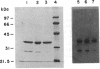
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong K. A., Acosta R., Ledner E., Machida Y., Pancotto M., McCormick M., Ohtsubo H., Ohtsubo E. A 37 X 10(3) molecular weight plasmid-encoded protein is required for replication and copy number control in the plasmid pSC101 and its temperature-sensitive derivative pHS1. J Mol Biol. 1984 May 25;175(3):331–348. doi: 10.1016/0022-2836(84)90352-8. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Raivio K. O., Bakay B., Adams W. B., Nyhan W. L. Variant human phosphoribosylpyrophosphate synthetase altered in regulatory and catalytic functions. J Clin Invest. 1980 Jan;65(1):109–120. doi: 10.1172/JCI109640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower S. G., Harlow K. W., Switzer R. L., Hove-Jensen B. Characterization of the Escherichia coli prsA1-encoded mutant phosphoribosylpyrophosphate synthetase identifies a divalent cation-nucleotide binding site. J Biol Chem. 1989 Jun 15;264(17):10287–10291. [PubMed] [Google Scholar]
- Bower S. G., Hove-Jensen B., Switzer R. L. Structure of the gene encoding phosphoribosylpyrophosphate synthetase (prsA) in Salmonella typhimurium. J Bacteriol. 1988 Jul;170(7):3243–3248. doi: 10.1128/jb.170.7.3243-3248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaterman K. B., Rosenberg G. H., Käufer N. F. Double-stranded sequencing, using mini-prep plasmids, in eleven hours. Biotechniques. 1988 Nov-Dec;6(10):951–952. [PubMed] [Google Scholar]
- Gibson K. J., Schubert K. R., Switzer R. L. Binding of the substrates and the allosteric inhibitor adenosine 5'-diphosphate to phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. J Biol Chem. 1982 Mar 10;257(5):2391–2396. [PubMed] [Google Scholar]
- Grandoni J. A., Switzer R. L., Makaroff C. A., Zalkin H. Evidence that the iron-sulfur cluster of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase determines stability of the enzyme to degradation in vivo. J Biol Chem. 1989 Apr 15;264(11):6058–6064. [PubMed] [Google Scholar]
- Hove-Jensen B. Cloning and characterization of the prs gene encoding phosphoribosylpyrophosphate synthetase of Escherichia coli. Mol Gen Genet. 1985;201(2):269–276. doi: 10.1007/BF00425670. [DOI] [PubMed] [Google Scholar]
- Hove-Jensen B., Harlow K. W., King C. J., Switzer R. L. Phosphoribosylpyrophosphate synthetase of Escherichia coli. Properties of the purified enzyme and primary structure of the prs gene. J Biol Chem. 1986 May 25;261(15):6765–6771. [PubMed] [Google Scholar]
- Hove-Jensen B. Mutation in the phosphoribosylpyrophosphate synthetase gene (prs) that results in simultaneous requirements for purine and pyrimidine nucleosides, nicotinamide nucleotide, histidine, and tryptophan in Escherichia coli. J Bacteriol. 1988 Mar;170(3):1148–1152. doi: 10.1128/jb.170.3.1148-1152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove-Jensen B., Nygaard P. Phosphoribosylpyrophosphate synthetase of Escherichia coli, Identification of a mutant enzyme. Eur J Biochem. 1982 Aug;126(2):327–332. doi: 10.1111/j.1432-1033.1982.tb06782.x. [DOI] [PubMed] [Google Scholar]
- Hove-Jensen B. Phosphoribosylpyrophosphate (PRPP)-less mutants of Escherichia coli. Mol Microbiol. 1989 Nov;3(11):1487–1492. doi: 10.1111/j.1365-2958.1989.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Jochimsen B. U., Hove-Jensen B., Garber B. B., Gots J. S. Characterization of a Salmonella typhimurium mutant defective in phosphoribosylpyrophosphate synthetase. J Gen Microbiol. 1985 Feb;131(2):245–252. doi: 10.1099/00221287-131-2-245. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nilsson D., Hove-Jensen B., Arnvig K. Primary structure of the tms and prs genes of Bacillus subtilis. Mol Gen Genet. 1989 Sep;218(3):565–571. doi: 10.1007/BF00332425. [DOI] [PubMed] [Google Scholar]
- Pandey N. K., Switzer R. L. Mutant strains of Salmonella typhimurium with defective phosphoribosylpyrophosphate synthetase activity. J Gen Microbiol. 1982 Aug;128(8):1863–1871. doi: 10.1099/00221287-128-8-1863. [DOI] [PubMed] [Google Scholar]
- Roessler B. J., Bell G., Heidler S., Seino S., Becker M., Palella T. D. Cloning of two distinct copies of human phosphoribosylpyrophosphate synthetase cDNA. Nucleic Acids Res. 1990 Jan 11;18(1):193–193. doi: 10.1093/nar/18.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd K. E., Menzel R. his operons of Escherichia coli and Salmonella typhimurium are regulated by DNA supercoiling. Proc Natl Acad Sci U S A. 1987 Jan;84(2):517–521. doi: 10.1073/pnas.84.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schubert K. R., Switzer R. L., Shelton E. Studies of the quaternary structure and the chemical properties of phosphoribosylpyrophosphate synthetase from Salmonella typhimurium. J Biol Chem. 1975 Sep 25;250(18):7492–7500. [PubMed] [Google Scholar]
- Switzer R. L., Gibson K. J. Phosphoribosylpyrophosphate synthetase (ribose-5-phosphate pyrophosphokinase) from Salmonella typhimurium. Methods Enzymol. 1978;51:3–11. doi: 10.1016/s0076-6879(78)51003-3. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. 3. Kinetic studies of the reaction mechanism. J Biol Chem. 1971 Apr 25;246(8):2447–2458. [PubMed] [Google Scholar]
- Switzer R. L., Sogin D. C. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. V. Inhibition by end products and regulation by adenosine diphosphate. J Biol Chem. 1973 Feb 10;248(3):1063–1073. [PubMed] [Google Scholar]
- Taira M., Ishijima S., Kita K., Yamada K., Iizasa T., Tatibana M. Nucleotide and deduced amino acid sequences of two distinct cDNAs for rat phosphoribosylpyrophosphate synthetase. J Biol Chem. 1987 Nov 5;262(31):14867–14870. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wild J., Walczak W., Krajewska-Grynkiewicz K., Klopotowski T. D-amino acid dehydrogenase: the enzyme of the first step of D-histidine and D-methionine racemization in Salmonella typhimurium. Mol Gen Genet. 1974;128(2):131–146. doi: 10.1007/BF02654486. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]