Abstract
Wound healing is a complex and orchestrated process that re-establishes the barrier and other functions of the skin. While wound healing proceeds apace in healthy individual, bacterial overgrowth and infection disrupts this process with significant morbidity and mortality. As such, any artificial matrix to promote wound healing must also control infecting microbes. We had earlier developed a two-part space-conforming gel backbone based on polyethyleneglycol (PEG) or lactose, which used ionic silver as the catalyst for gelation. As silver is widely used as an in vitro antimicrobial, use of silver as a catalyst for gelation provided the opportunity to assess its function as an anti-microbial agent in the gels. We found that these gels show bacteriostatic and bactericidal activity for a range of Gram-negative and Gram-positive organisms, including aerobic as well as anaerobic bacteria. This activity lasted for days, as silver leached out of the formed gels over a day in the manner of second-order decay. Importantly the gels did not limit either cell growth or viability, though cell migration was affected. Adding collagen I fragments to the gels corrected this effect on cell migration. We also found that the PEG gel did not interfere with hemostasis. These observations provide the basis for use of the gel backbones for incorporation of anesthetic agents and factors that promote wound repair. In conclusion, silver ions can serve dual functions of catalyzing gelation and providing anti-microbial properties to a biocompatible polymer.
Keywords: Wound healing, Infections, Bacteria, Hemostasis
1. Introduction
Wound healing is a dynamic and orchestrated process that involves cellular and matrix components acting together to re-establish the lost tissues [1,2]. Co-morbid physiological and psychological conditions and aging compromise this healing, which can be ameliorated by modulating these underlying conditions (with the exception of aging). Infections adversely impact wound repair through ongoing chronic inflammation and production of toxic molecules and metabolites from both the microbe and the immune response [3]. Skin wounds are particularly prone to these infections as they are both exposed to pathogens and also harbor commensural microbes that can infect compromised tissue. Thus microbial proliferation must be controlled or prevented to enable proper healing [4].
In designing a material or matrix to promote wound healing, one that is capable of providing adequate antimicrobial activity is desired provided that the anti-microbial substance present in it does not compromise the physiologic healing, including hemostasis and immune functioning and the repair-promoting physiochemical aspects of the matrix [5]. To be beneficial, the antimicrobial agent must also exert its effect over the relevant time scale of days, without being washed out by tissue fluid flows or neutralized by serum-derived and tissue-derived factors and enzymatic activities [5].
With the surge of antibiotic resistance, impregnating a matrix with a necessarily broad-spectrum antibiotic is becoming a concern nowadays [6]. Moreover, complex molecules may form complexes with the surface of polymer and modify it further resulting in confounding diffusion and elution calculations. This also may produce novel immunological epitopes that would be recognized as foreign. For such reasons, silver is a widely used nonspecific antimicrobial, as it acts against a very broad spectrum of bacterial, yeast and fungal species likely to contaminate wounds and body cavities [7]. This action derives from the binding of the positive silver ions with the negatively charged microbial proteins preventing their replication, and via attachment to sulfhydryl groups preventing their respiration resulting in inhibiting their proliferation [8-10]. Silver also works on biofilms, a critical challenge for embedded foreign bodies [11].
With the above considerations, we have developed a wound-healing matrix which is space-forming, biocompatible and degradable over a 2-week-long period. However, these self-curing polymers based on lactose or polyethylene glycol (PEG) along with 3,4 dihydroxyphenyl-L-alanine (l-DOPA), Lysine backbones could represent foreign bodies, which may form nidi for infection [12]. These polymers are produced in two parts with gelation being catalyzed by ionic silver (Ag) with peroxydiphosphate. This mode of catalysis was chosen as silver is widely used as an antimicrobial [13-15] and would distribute through the matrix passively. The other core issues of being biocompatible to living tissues and having hemostatic property are also given importance considering wound repair. Thus, we asked if this matrix would limit the growth of bacteria and yeast that commonly infect skin wounds delaying the healing process.
2. Materials and methods
2.1. Materials
All reagents in the polymer gel namely PEG, l-DOPA, lactose (Lac), silver (Ag), peroxydiphosphate were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Lysine diisocyanate methyl ester (LDI) was obtained from Chemical Division, Kyowa Hakko Kogyo Co. Ltd. (Tokyo, Japan). Tissue culture medium was from Life Technologies (Grand Island, NY, USA).
Ten microorganisms commonly associated with human skin or wound infections were selected as a challenge. The microorganisms were obtained either from ATCC (Manassus, VA, USA) or from human isolates from the Clinical Microbiology Laboratory of the UPMC Presbyterian/Shady-side Hospital; except Eschericia coli and Acinetobacter baumanii which were recovered from an actual human wound. Micrococcus luteus, Staphylococcus aureus (ATCC 25923), Streptococcus pyogenes, Diphtheroids, Pseudomonas aeruginosa (ATCC 27853), Clostridium perfringens, Bacillus subtilis, Candida albicans comprise the other eight out of a total of ten organisms tested. The use of PHI-independent clinical isolates and human cells were approved as exempted by the University of Pittsburgh IRB.
2.2. Synthesis of polymers
2.2.1. Synthesis of lactose-(LDI–DOPA)8 prepolymer (DLL)
Glassware was dried before use. Lactose 0.70 g (FW 342.3; 2 mmol, –OH 16 mmol) dissolved in 5 ml of dimethyl sulfoxide (DMSO) in a round bottomed flask was flushed with nitrogen [1]. A rubber septum was used to seal the flask. Using a syringe 3.1 ml of LDI (FW 212, d 1.157, 16 mmol, –NCO 32 mmol) was added to the flask. All the constituents in the flask were stirred in the dark at room temperature for 2 days. FT-IR and 1H NMR were used in monitoring the isocyanate group consumption and formation of urethane linkages. A solution of DOPA (3.2 g in 20 ml DMSO, FW 197, 16 mmol) was added to the constituents in the flask when the FT-IR showed that 50% of isocyanate groups (peak at 2266 cm−1) which were initially present had been consumed. The constituents in the flask were stirred at room temperature for another three days until the FT-IR showed that no more isocyanate groups were present. The product obtained were transferred to dialysis tubing (molecular weight cutoff 1000, Spectrum Laboratories, Inc., Fort Lauderdale, FL, USA) and dialyzed against three changes of distilled deionized water (DDI water) and dried under vacuum at room temperature. The molecular weight of the product was determined by mass spectrometry. The net DLL pre-polymer obtained was 85.3% pure.
2.2.2. Synthesis of PEG-(LDI–DOPA)2 prepolymer (DLP)
DLP was synthesized in the same manner as above [1]. In brief 2 g of PEG (FW 400, 10 mmol, –OH groups) were reacted with 2 ml of LDI (10 mmol, –NCO 20 mmol) for 2 days. Subsequently 2 g DOPA (FW 197, 10 mmol, –NH2 10 mmol) in 20 ml of DMSO was added and reacted at room temperature for 3 days. The reaction mixture was dialyzed against water and dried under vacuum. The net DLP prepolymer obtained was 81.5% pure.
2.2.3. Synthesis of PEG-(LDI–DOPA)2 prepolymer (DLP) with collagen
Glassware was dried in a vacuum oven prior to use. 2 g PEG were reacted with 2 ml of LDI (FW 226, d 1.157, 10.75 mmol, –NCO 21.5 mmol) for two days, then 2 g DOPA in 20 ml of DMSO were added (at this point, the reaction mixture was not clear). The reaction was performed at room temperature for three days until the reaction mixture was clear and FT-IR showed no remaining isocyanate groups. Then, N-hydroxysuccinimide (1.15 g, 10 mmol) and 2.06 g dicyclohexylcarbodiimide were added. The reaction mixture was stirred at room temperature for 20 h. Dicyclohexylurea was removed by centrifugation (3000 rpm for 30 min). The supernatant was transferred to a flask and 0.1 g collagen I (from Bovine) was added. The reaction mixture was stirred at room temperature for 10 h, then, transferred to dialysis tubing (Spectrum Laboratories, Inc., Fort Lauderdale, FL, USA, molecular weight cutoff of 1000). The products were dialyzed against DDI water to remove DMSO and small molecular weight monomers (water changed every three hours) until a precipitate appeared. The precipitate was dissolved with 0.1 m Na2B4O7 and the solution was initiated by AgNO3/K4P2O8 to make gel.
2.2.4. Preparation of the polymer gels
The DOPA containing polymer gels were prepared by radical polymerization of an aqueous solution of prepolymer using the redox initiation system Ag+/potassium peroxydiphosphate [1]. Fifty microliters of AgNO3/K4P2O8 solution (various loadings) were added to 7 ml of prepolymer solution (0.3 g prepolymer dissolved in 1 ml of 0.1 m Na2B407 solution) in a 75 mm Teflon dish.
2.2.5. Polymer degradation in vitro
Polymer degradation was tested in vitro by placing known amounts of polymer gel in phosphate buffer solution (1 × PBS; 100 mg polymer/ml PBS) at 37 °C for up to 3 weeks [1]. The concentration of DOPA liberated from the polymer was detected by UV-VIS as described by Waite and Benedict [16]. The changes in pH of the solution due to polymer degradation were assessed in parallel samples with the use of a pH meter.
2.2.6. Swelling property of polymer gels
The determination of swelling characteristics of polymer gels was determined at 37 °C using a gravimetric method [17,18]. The dry gels were weighed at first and then immersed in a phosphate buffer solution at a pH 7.4. At different time points the swollen gels were removed from the buffer and weighed on a sensitive balance. The swelling ratio was calculated as follows:
where Ws and Wd stand for swollen and dry weights of the gels, respectively.
The change in the diameter of the gel at various time points was also determined.
2.3. Microbial analysis
2.3.1. Disc diffusion assay
Initially, we tested the antimicrobial properties by a modification of the disc diffusion method. This provided a measure of both the antimicrobial activity and diffusibility of the gelated Ag [19].
The control ‘disc’, was filter paper (Schleicher and Schuell Co., Keene, NH, USA), 1.25 cm diameter size and 1 mm thickness, which was impregnated with 20 μl of 1% silver nitrate solution for a final mass of 0.125 μg Ag. Test ‘discs’ were cut from polymerized matrix, 2 cm diameter in size and 1 mm in thickness. In the prepolymer solution we had 10 μg/μl of Ag for a final load of 3.3 μg/disc.
The stock microbial cultures were plated onto the blood agar plates and colonies were picked manually. They were suspended in trypticase soy broth (BD Diagnostics, Sparks, MD, USA) to obtain an equivalent to 0.5 Mcfarland barium sulfate (107–108 CFU/ml) standard. A sterile cotton swab was suspended in the microbe-containing trypticase soy broth and used to streak at right angles in four directions in blood agar plates to form a confluent lawn of growth on 150 mm plates of Mueller-Hinton agar with sheep blood (BD Diagnostics, Sparks, MD, USA). Test and control discs were placed on the plates which were incubated at 37 °C for 24 h. The zone of inhibition surrounding the control as well as our experimental gels was measured. For anaerobic organisms the inoculated plates were placed into a sealed anaerobic bag (AnaBag 150) (Hardy Diagnostics, Santa Maria, CA, USA) containing an anaerobic generator sachet (Anaerogen) (Gibson Laboratories Inc., Lexington, KY, USA). The anaerobic cultures were incubated for 48 h at 25 °C. For each experiment, a similar plate inoculated with Micrococcus luteus served as a control.
2.3.2. Microbicidal and microbiostatic assays
To distinguish whether the microbial inhibitions noted in the disc diffusion method were due to bactericidal or bacteriostatic effects, quantitative assays were employed taking into consideration the aerobes, anaerobes and yeast [19-21].
The suspension of the microorganism was made by first allowing the organism to grow in appropriate broth. For all the microorganisms except S. pyogenes, S. aureus and C. perfringens, Mueller Hinton Broth (BD Diagnostics, Sparks, MD, USA) was used. For S. pyogenes and S. aureus Todd Hewitt Broth (BD Diagnostics, Sparks, MD, USA) was used. For C. perfringens trypticase soy broth was being used. The growth was adjusted to a 0.5 Mcfarland Standard (107–108 CFU/ml) and a 1/200 dilution of this broth was made (105–108 CFU/ml) using saline as a diluent. For each assay, four tubes, each containing 4 ml of broth were used. One tube contained one disc of the gel under test which was 1.3 cm in diameter and 1 mm in thickness with the final load of Ag being 2.58 μg. The second tube contained two discs of the gel, a third tube contained three discs. The fourth tube contained no gel and served as a growth control. To each of the four tubes, 0.1 ml of the diluted bacterial suspension was added.
For C. perfringens, a 1/100 dilution of the standardized suspension was used as the inoculum. Aerobes were incubated at 37 °C for 24 h whereas anaerobes were incubated at 25 °C for 48 h. Before incubation, the actual size of the inoculum was determined by making serial dilutions from the inoculated growth control tube. One-tenth ml of these dilutions were inoculated in triplicate onto blood agar plates (BD Diagnostic Systems, Sparks, MD, USA) which were incubated overnight at 37 °C. After incubation of the tubes, similar dilutions were made and plated to determine the fraction of the original inoculum that survived.
2.4. Atomic absorption spectroscopy
Quantitative measurement of the Ag both retained in and leaving from the gel would suggest temporal nature of the antimicrobial activities [22,23]. Total leaching of Ag from gels was assessed by placing a 1.3 cm diameter, 1 mm thick piece of gel in trypticase soy broth for 10 days having a final load of Ag 2.58 μg. In addition, the daily leaching of silver was assessed by moving gels from tube to tube of trypticase soy broth at 24 h intervals. Ag concentration was determined by atomic absorption spectroscopy using a Perkin–Elmer EDLS (Perkin Elmer AA5100, Wellesley, MA, USA).
2.5. Dermal fibroblast and keratinocyte functioning
The gels were assessed for biocompatibility by determining effects on survival, proliferation and migration of human dermal cells and keratinocytes [1]. Primary human dermal fibroblasts were obtained from Musculoskeletal Research Center, Department of Orthopedic Surgery, University of Pittsburgh Medical center, Pittsburgh, PA, USA. For human keratinocytes we used HaCaT cells, a spontaneously immortalized, but not transformed human keratinocyte cell-line which was provided by Dr. N. Fusenig German Cancer Research Center (Heidelberg, Germany). These studies were deemed exempted (4e) by the University of Pittsburgh IRB.
The primary human dermal fibrobasts and keratinocytes were subcultured into 24 well plates at 20,000 per well. When the cells became 70% confluent the gel additive was placed along with 1 ml of complete medium. Solid gels were 1 mm thick and cut to 90% of the well surface (9 mm diameter); Liquid gels were of the same solution used for the solid gel but for the initiators, and added to cover the well surface completely. Cell viability was assessed at 24, 48 and 72 h by MTT Assay and cell proliferation by automated Coulter Counter (Beckman Coulter Instruments). For cell migration, an in vitro wound-healing assay was performed.
2.6. Hemostasis assessments
As the gel matrix will be placed into wounds with blood, it needs to be either hemostatic or not interfere with the normal hemostasis [24]. Excess human blood, citrated blood and platelet-poor plasma, were used as approved by the University of Pittsburgh IRB (exemption 4e). Prothrombin time, activated partial thromboplastin time, clotting time were measured by using tube method with reagents from BioData Corp. (Horsham, PA, USA).
3. Results
3.1. The silver-catalyzed gels present antimicrobial activities
Both the lactose and PEG discs demonstrated inhibition of the growth of M. luteus and all of the test organisms (Table 1). The PEG gel presented larger zones of inhibition with one exception. However, both gels provided for greater inhibition than the Ag-impregnated filter paper.
Table 1.
Zones of inhibition (in mm) obtained with control and gels of either type as determined by the disc diffusion method
| Microorganisms | Control (1%AgNO3) | PEG, LDI, DOPA, Ag, peroxydiphosphate |
Lactose, LDI, DOPA, Ag, peroxydiphosphate |
|---|---|---|---|
| M. luteus | 18 | 50 | 22 |
| S. aureus | 15 | 36 | 21 |
| S. pyogenes | 14 | 36 | 22 |
| Diphtheroids | 15 | 23 | 31 |
| P. aeruginosa | 13.5 | 37 | 22 |
| E. coli | 15 | 35 | 21 |
| Acinetobacter | 16 | 33 | 27 |
| C. perfringens | 14 | 25 | 23 |
| B. subtilis | 13 | 35 | 21 |
| C. albicans | 16 | 38 | 23 |
The microorganisms were streaked on individual blood agar plates. Control consisted of 1% AgNO3. PEG containing gel had PEG, LDI, DOPA, Ag, peroxydiphosphate and lactose containing gel had lactose, LDI, DOPA, Ag, peroxydiphosphate. Plugs for each gel type had silver concentration of 3.3 μg, diameter of 2 cm and thickness of 1 mm. Control consisted of filter paper disc with a diameter of 1.25 cm and thickness of 1 mm.
To determine the nature of the inhibition we assessed microbicidal and static capacities as accomplished by one to three gel discs. The sensitivities of the agents varied (Table 2). A 3-log reduction in microbe number considering its initial count was taken as bactericidal activity. For all aerobic organisms significant antimicrobial activity was seen in the first 24 h with both gels. Both the gels displayed a concentration dependant bactericidal action with E. coli and P. aeruginosa with more than one gel disc and bacteriostatic effect with one gel disc. With A. baumanii a time dependant antimicrobial activity was seen. All Gram-positive bacteria had similar time dependant kinetics. With S. aureus a 2-log reduction at 24 h was seen when challenged with both gels. S. pyogenes and Diphtheroids were similar in behavior considering S. aureus except when challenged with three gel discs of either type where in a 3-log reduction was seen. With C. albicans the effect was found similar as with Gram-positive bacteria. With the anaerobes a time dependant antimicrobial activity was seen at 48 h with both the gels.
Table 2.
Decrements in colony count upon challenge by gels
| Organism | Number of gel discs per tube | PEG containing gel |
Lactose containing gel |
||||
|---|---|---|---|---|---|---|---|
| Bacterial concentration at time (CFU/ml) |
Bacterial concentration at time (CFU/ml) |
||||||
| 0 h | 24 h | 48 h | 0 h | 24 h | 24 h | ||
| S. aureus | 1 Disc | 2.5 × 105 | 1.86 × 103 | 1.72 × 103 | 2.5 × 105 | 2.18 × 103 | 2.06 × 103 |
| 2 Discs | 1.05 × 103 | 9.06 × 102 | 1.96 × 103 | 1.55 × 103 | |||
| 3 Discs | 4.80 × 102 | 4.25 × 102 | 1.01 × 103 | 9.05 × 102 | |||
| S. pyogenes | 1 Disc | 2.27 × 105 | 1.49 × 103 | 1.19 × 103 | 2.27 × 105 | 1.79 × 103 | 1.48 × 103 |
| 2 Discs | 1.01 × 103 | 6.05 × 102 | 1.19 × 103 | 7.95 × 102 | |||
| 3 Discs | 2.55 × 102 | 1.55 × 102 | 6.00 × 102 | 3.65 × 102 | |||
| Diphtheroid | 1 Disc | 2.33 × 105 | 5.80 × 102 | 4.25 × 102 | 2.33 × 105 | 8.45 × 102 | 6.85 × 102 |
| 2 Discs | 2.10 × 102 | 90 | 5.35 × 102 | 3.90 × 102 | |||
| 3 Discs | 9 | 15 | 3.10 × 102 | 2.35 × 102 | |||
| P. aeruginosa | 1 Disc | 2.28 × 105 | 5.95 × 102 | 1.80 × 102 | 2.28 × 105 | 8.20 × 102 | 3.85 × 102 |
| 2 Discs | 0 | — | 0 | — | |||
| 3 Discs | 0 | — | 0 | — | |||
| E. coli | 1 Disc | 2.32 × 105 | 1.36 × 103 | 8.95 × 102 | 2.28 × 105 | 1.98 × 103 | 1.52 × 103 |
| 2 Discs | 0 | — | 0 | — | |||
| 3 Discs | 0 | — | 0 | — | |||
| A. baumanii | 1 Disc | 2.35 × 105 | 9.15 × 102 | 7.85 × 102 | 2.35 × 105 | 1.13 × 103 | 9.80 × 102 |
| 2 Discs | 5.70 × 102 | 4.45 × 102 | 7.65 × 103 | 6.45 × 102 | |||
| 3 Discs | 2.55 × 102 | 1.75 × 102 | 5.05 × 102 | 3.75 × 102 | |||
| C. perfringens | 1 Disc | 2.38 × 105 | 1.02 × 103 | 2.38 × 105 | 1.39 × 103 | ||
| 2 Discs | 7.10 × 102 | 9.06 × 102 | |||||
| 3 Discs | 3.85 × 102 | 6.05 × 102 | |||||
| C. albicans | 1 Disc | 2.33 × 105 | 5.80 × 102 | 4.40 × 102 | 2.33 × 105 | 8.30 × 102 | 7.30 × 102 |
| 2 Discs | 3.00 × 102 | 2.00 × 102 | 4.65 × 102 | 3.85 × 102 | |||
| 3 Discs | 1.55 × 102 | 35 | 2.30 × 102 | 1.70 × 102 | |||
Target microorganisms were seeded at 105 CFU/ml. Parallel tubes were seeded with one to three gels. At 24 and 48 h the CFU/ml were determined. Anaerobic C. perfringens was counted only at 48 h considering their normal growth patterns. Shown are the bacterial counts in CFU/ml. Placement of a control tube having microorganisms at 105 at 0 h gave non log growth counts at 24 and 48 h for aerobes and 48 h for anaerobes (data not shown). The composition of both gels are the same as described in earlier table.
3.2. Silver leaches from the gels over an extended time period
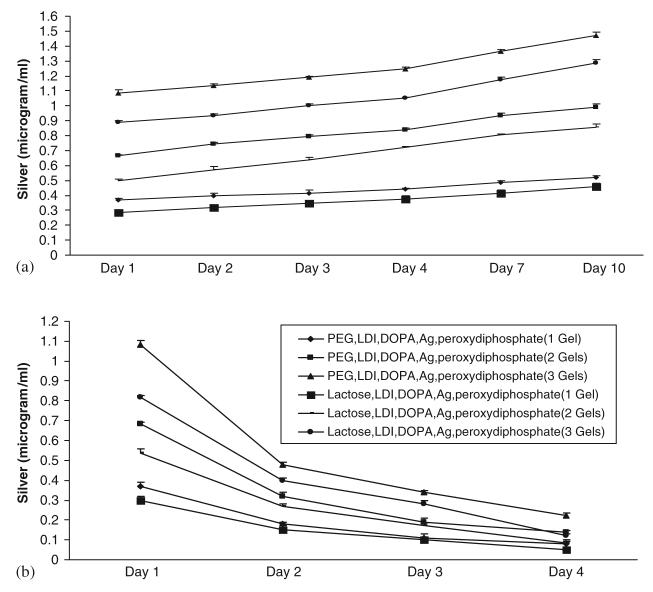
Continued presence of Ag would be needed to keep a wound bed uninfected, while retention in the gel is required to prevent a foreign body nidus of infection. Ag was detected in the media increasingly with time (Fig. 1(a)). The Ag rapidly leaves the PEG gel with 57% being detected in the media in 24 h. This increases to 68% at day 4 and 80% by day 10. Leaching from the lactose gel was lesser considering PEG gel with only 44% after one day, increasing to 58% at 4 days and 71% at 10 days.
Fig. 1.
Silver leaches out of the gels over several days as determined by atomic absorption spectroscopy. (a) Upper graph shows cumulative silver concentration over a period of 10 days in broth in which the gels were immersed (one to three gels were tested). (b) Lower graph shows silver concentrations in the broth for tubes that were seeded between one and three gels and switched to fresh broth daily for 4 days.
As can be seen, Ag dissemination from the gels appears to persist for days. When assessed directly, we found that the second day saw 28% of silver leave PEG gels and 23% of lactose gels, respectively (Fig. 1(b)). This dropped subsequently but was still evident on day 4, with 12% and 7% leaving, respectively. This greater leaching from gels placed in fresh media would be consistent with passive diffusion being the driving force of Ag distribution.
3.3. Polymer degradation
To be applicable as a wound-healing matrix, the cured polymer needs to remain intact over a number of days. Polymer degradation was tested in vitro by placing 100 mg of polymer into 1 ml of phosphate buffer solution at 37 °C and changing the buffer daily. The rate of degradation was monitored by measuring the amount of DOPA released from the polymer gel as a function of time. As a 1,2-benzenediol, DOPA exhibits a maximum UV absorbance in aqueous solution at approximately 280 nm (Fig. 2(a)). Since lysine, lactose and PEG do not absorb significantly at this wavelength, the concentration of DOPA was determined by UV absorbance at 280 nm. The standard curve of DOPA is shown in Fig. 2(b).
Fig. 2.
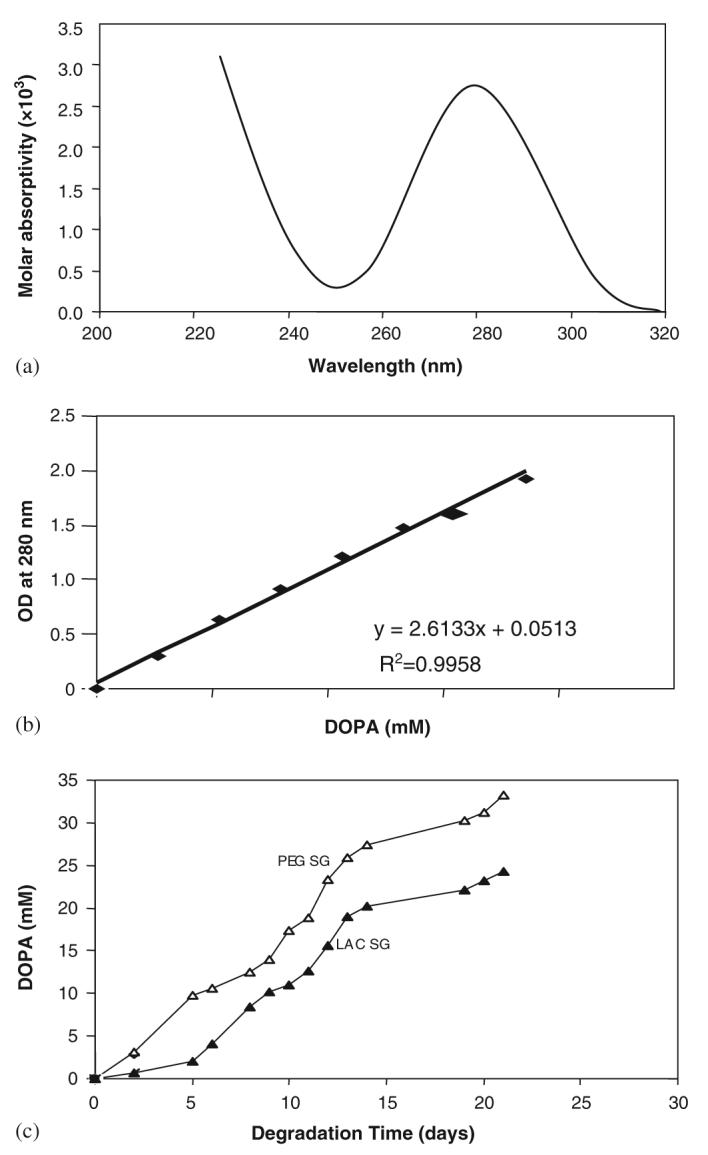
(a) UV spectrum of DOPA with maximal absorbance in aqueous solution at 280 nm. (b) The standard curve of DOPA was obtained by the determination of OD at 280 nm and the correlation coefficient of the standard curve was 0.9958. (c) Degradation of various DOPA-containing polymer gels in PBS. The polymer gels were incubated in PBS for 3 weeks and the degradation was tested by the concentration of DOPA released from the polymer gel into the solution.( PEG SG-solid gel with PEG, LDI, DOPA, Ag and peroxydiphosphate; LAC LG- liquid gel with lactose, LDI, DOPA, Ag and peroxydiphosphate).
Degradation testing indicated that the DOPA-containing polymer was degraded over a period of 3 weeks. Further, as shown in Fig. 2(c) using gels formed from mixtures of lactose and PEG based prepolymers, the time required to achieve a certain extent of degradation increases as the functionality of the network increases. We measured the pH of the PBS containing 100 mg/ml polymer over a period of 21 days, the degradation products of the DOPA-containing polymers (PEG gel and lactose gel) had no significant effect on the pH of polymer degradation solution at physiological temperature tested (data not shown). These data suggest that a functional polymer can be generated from these materials.
3.4. Swelling property of polymer gels
PEG or lactose containing polymer gels exhibited hydrophilic characteristics. As a consequence of that they were seen to swell up when placed in phosphate buffer solution at 37 °C. As seen in Fig. 3(a), the amount of uptake of water increased for the initial 30 min after which it attained its saturation point. This is to a degree reflective of drug release capabilities of a wound matrix. In Fig. 3(b), the change in diameter was also recorded to better understand the flexible nature of the polymer gels to its maximum extent. PEG gel had more hydrophilic character than lactose gel.
Fig. 3.
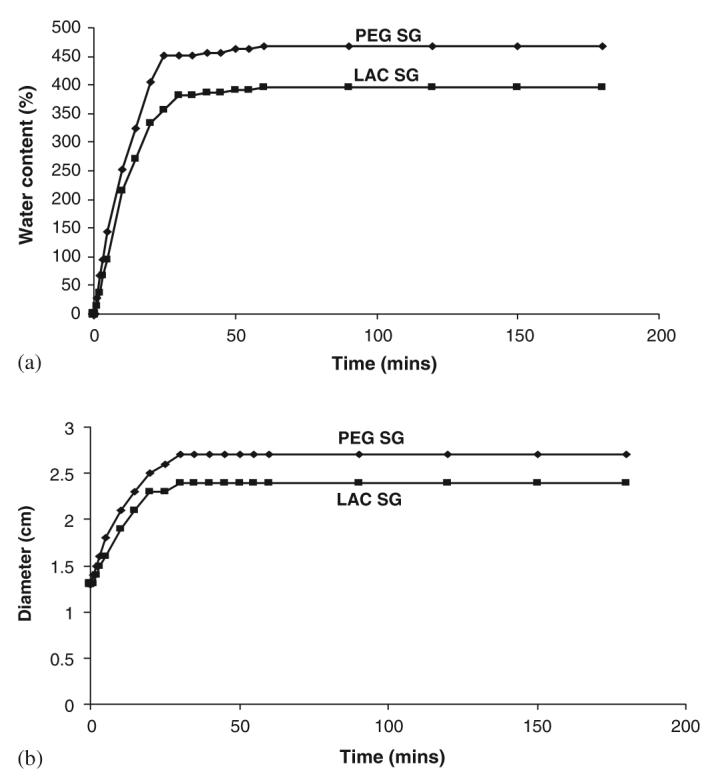
(a) Swelling property of the gels as a function of time at 37 °C. (b) Change in diameter of the gels as a function of time at 37 °C. (PEG SGPEG, LDI, DOPA, Ag, peroxydiphosphate; LAC SG-lactose, LDI, DOPA, Ag, peroxydiphosphate).
3.5. Cell viability and proliferation are not compromised by the gels
The gel when fully designed is to be placed in direct contact with the structural cells of skin, fibroblasts and keratinocytes, and as such needs to be nontoxic to these. Despite the Ag being broadly microbiocidal, these gels did not compromise either the viability or growth of the fibroblasts (Figs. 4(a) and (b)) [25,26] or keratinocytes (Figs. 5(a) and (b)) [26,27]. This was true both for gelated polymers (SG, solid gel) or those in which the catalysts were omitted (LG, liquid gels).
Fig. 4.
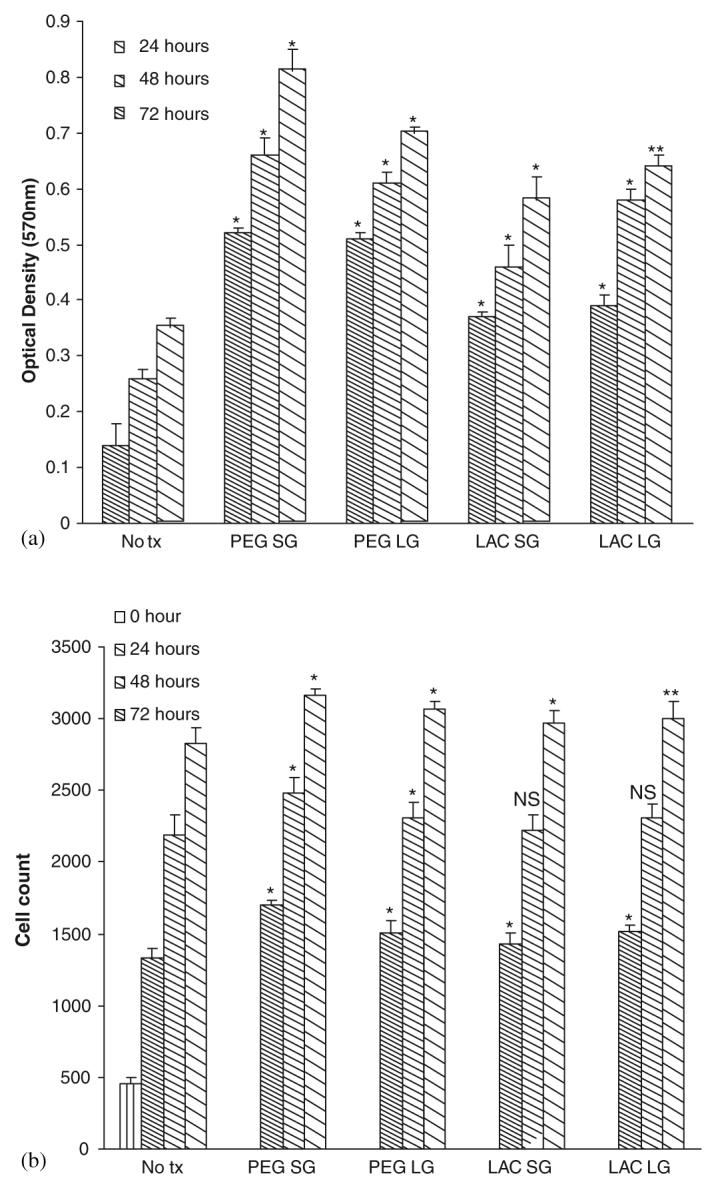
Effect of gels on human dermal fibroblasts: (a) cell viability and (b) cell proliferation. Cells were exposed to gels (PEG SG-solid gel with PEG, LDI, DOPA, Ag, and peroxydiphosphate; PEG LG-liquid gel with PEG, LDI, and DOPA; LAC SG-solid gel with lactose, LDI, DOPA, Ag, and peroxydiphosphate; LAC LG-liquid gel with lactose, LDI, and DOPA) after 48 h in serum-depleted media. Cell viability and proliferation were assessed at 24, 48 and 72 h. Values are expressed as mean ±SEM (n = 3). NS = not significant, *p<0.05, **p<0.001 compared to diluent alone (No tx-No treatment).
Fig. 5.
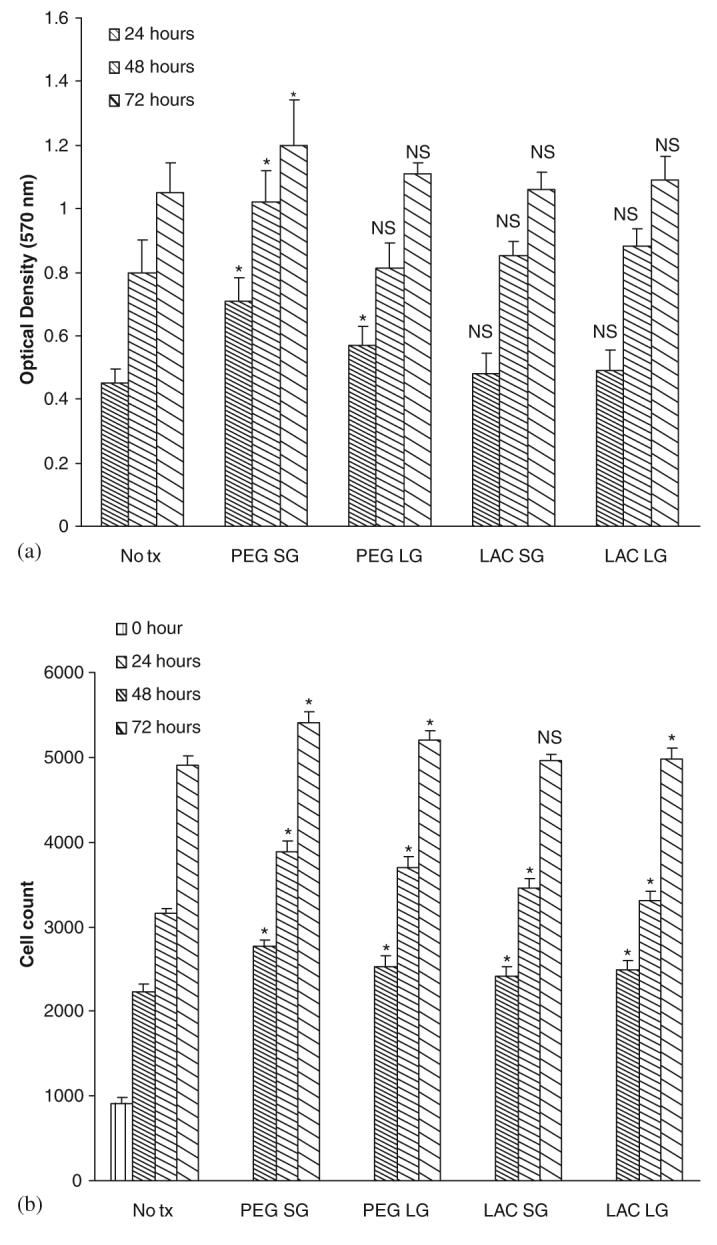
Effect of gels on human keratinocytes: (a) cell viability and (b) cell proliferation. Cells were exposed to gels with composition as described in previous figure legend after 48 h in serum-depleted media. Cell viability and proliferation were assessed at 24, 48 and 72 h. Values are expressed as mean ±SEM (n = 3). NS = not significant, * = p<0.05 compared to diluent alone (No tx).
3.6. Gel matrices limited cell migration which can be rescued by collagen
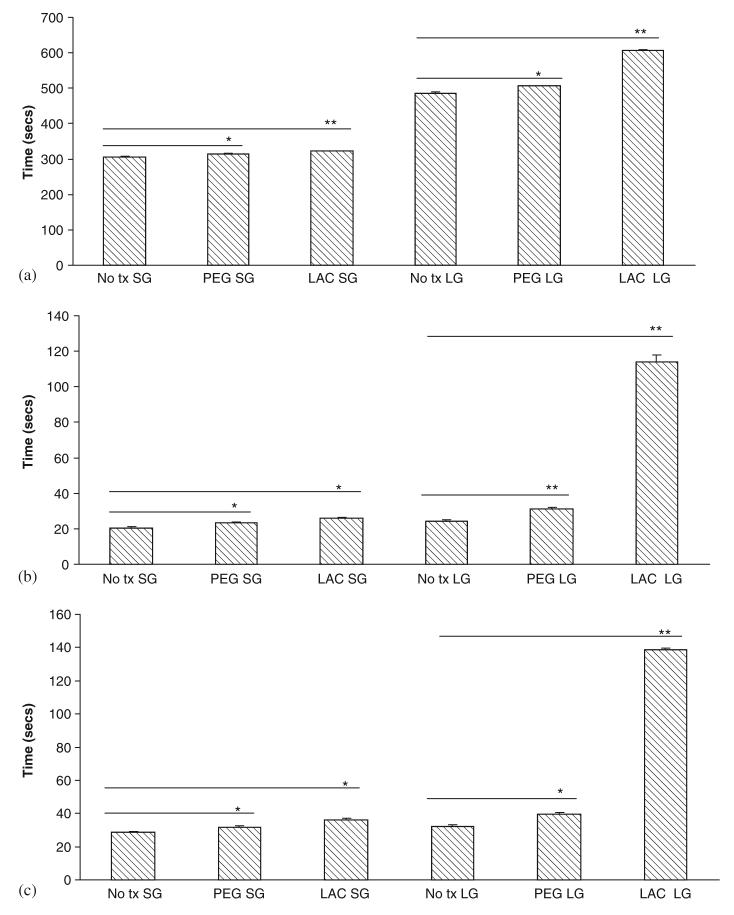
Wound healing requires active migration of the structural cells into the defect, and in the case of a wound gel, into the gel to replace this artificial matrix. Unfortunately, both gels limited the movement of both cell types (Figs. 6(a) and (b)). This limitation was noted with both gelated and liquid gels, suggesting that multiple factors adversely affected cell movement. This was confirmed by examining cell migration in the face of individual components, and finding that lactose, L-DOPA, and LDI all limited cell movement (Figs. 7(a) and (b)).
Fig. 6.
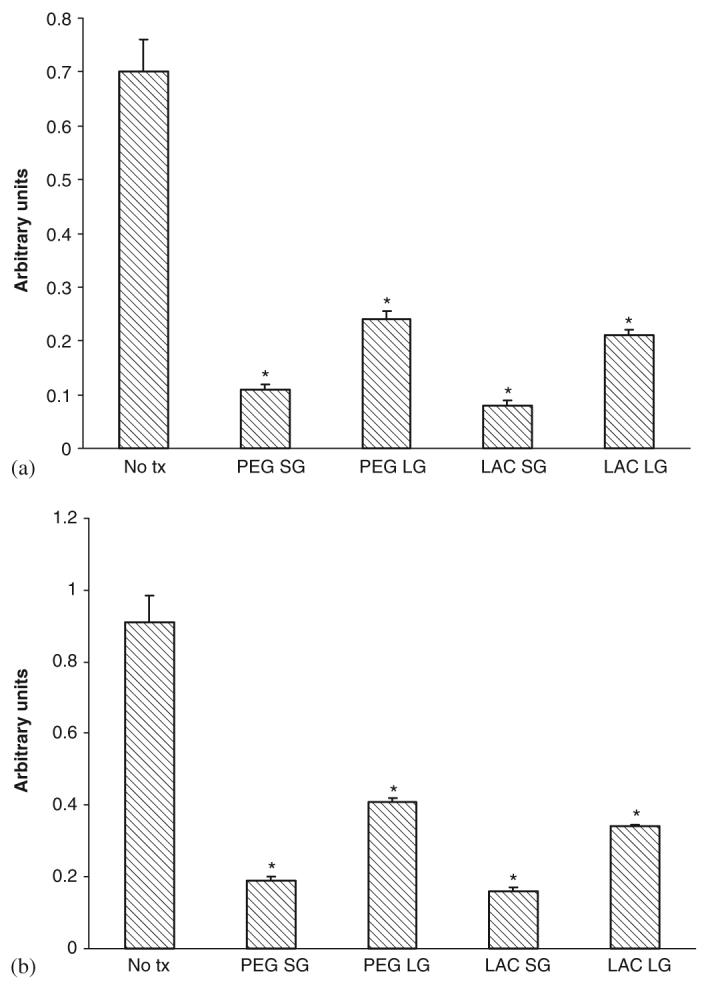
Effect of gels on (a) human dermal fibroblast and (b) keratinocyte cell motility. Gels composition was as described in previous figure legends. Cells were exposed to gels after 48 h in serum depleted media and motility assessed by an in vitro wound healing assay. Values are expressed as mean ±SEM (n = 3), *p<0.05 compared to diluent alone (No tx).
Fig. 7.
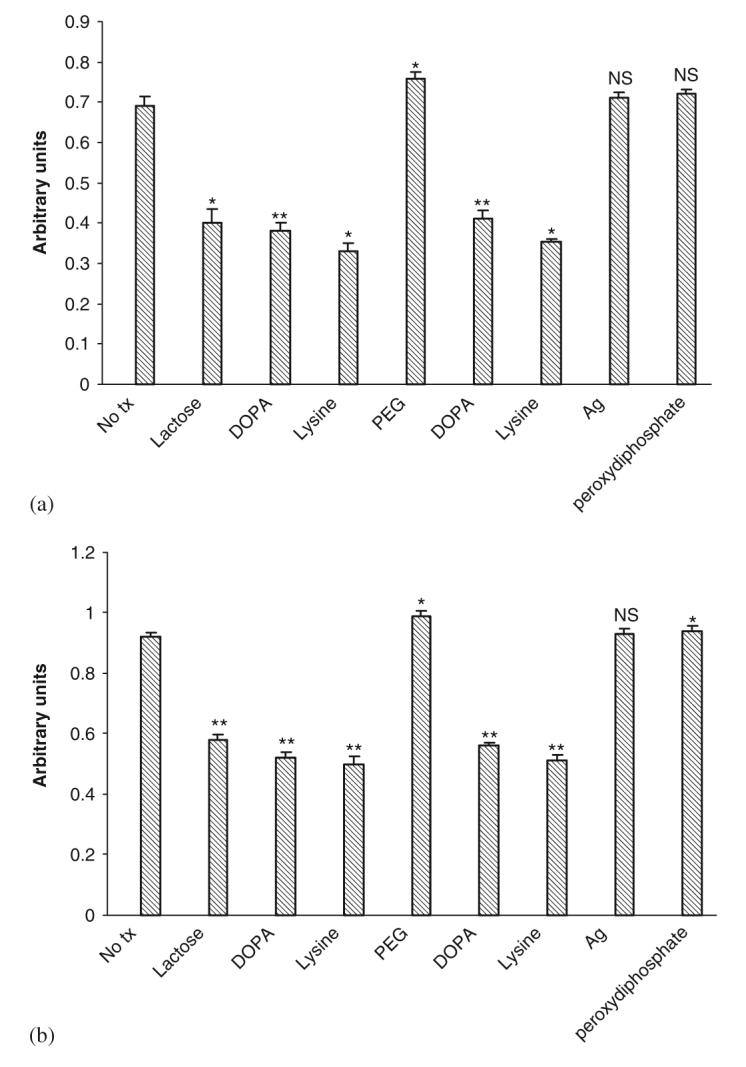
Effects of individual components of gel on (a) human dermal fibroblast and (b) keratinocyte cell motility. The cells were exposed to gels after 48 h treatment in serum depleted media and motility assessed by an in vitro wound healing assay. Values are expressed as mean ±SEM (n = 3), NS = not significant, *p<0.05, **p<0.01 compared to diluent alone (No tx).
We sought to correct this adverse impact on cell function. Rather than looking for replacements for the components identified above, we determined whether an additive would counter this effect because these components were as likely to be physiochemical as molecular and thereby not avoided by simple replacement approaches. Fibrillar collagen is a component of skin extracellular matrix, used clinically [28-30], and is amenable to industrial production parameters. Incorporation of collagen I fibers in the PEG gel compensated for the migration inhibition (Figs. 8(a) and (b)) resulting in movement statistically not different from that in the absence of any gel [31-33]. The lactose gel was not re-engineered due to issues described in the following sections.
Fig. 8.
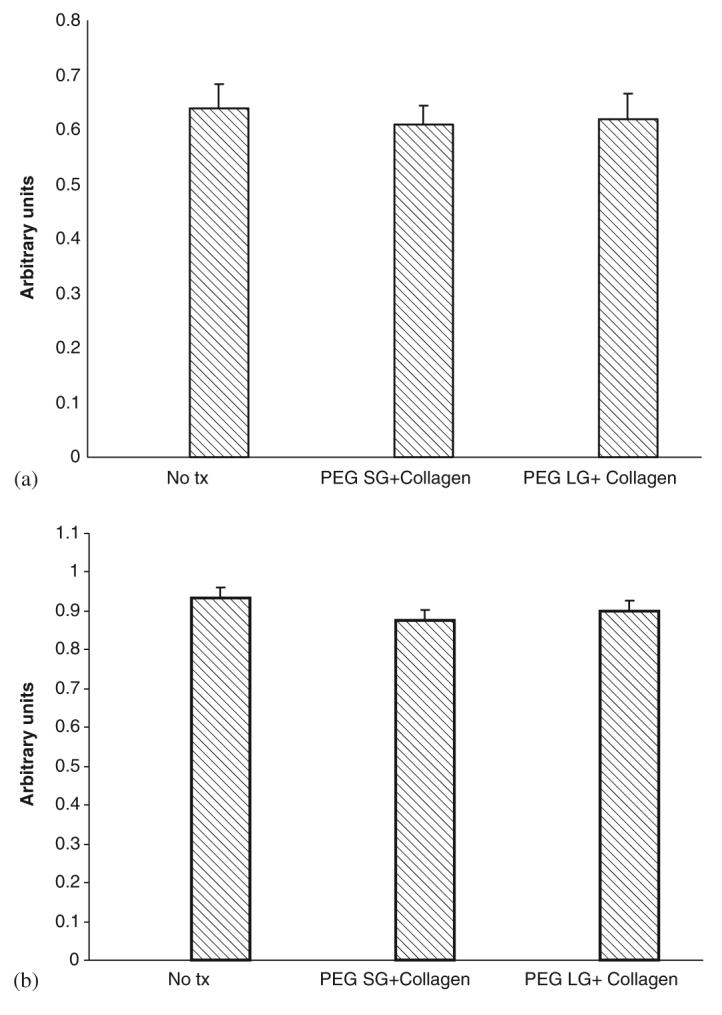
Effect of gels with collagen on (a) human dermal fibroblast and (b) keratinocyte cell motility. Cells were exposed to gels. PEG SG-solid gel having PEG, LDI, DOPA, Ag, and peroxydiphosphate; PEG LG-liquid gel having PEG, LDI, and DOPA; both with collagen after 48 h treatment in serum depleted media. Cell motility was assessed by in vitro wound healing assay. Values are expressed as mean±SEM (n = 3).
3.7. PEG gel does not compromise hemostasis but lactose gel does
As the gel is being designed to be placed in wound fields in which bleeding occurs, hemostasis must be enabled. The PT, aPTT, and clotting time of human blood were within the normal range in the presence of the solid PEG and lactose gels (Figs. 9(a)-(c)). However, the gel will be introduced into the wound in the pre-gelated liquid form and thus must be tested as such. While the liquid PEG gel demonstrated somewhat elongated hemostatic parameters, these were not different from the dilutional effect of a 25% volume addition (no tx LG), and are thus considered not a concern. The liquid lactose gel, however, did severely compromise all three hemostatic responses beyond the dilutional effect.
Fig. 9.
Effect of gels on (a) clotting time (b) prothrombin time and (c) partial thromboplastin time as assessed by the tube method. Gel composition was as described previously. Values are expressed as mean±SEM (n = 3), *p<0.05,**p<0.01. Bars above the graphs indicate the groups compared.
4. Discussion
We are developing a self-curing (gelation), space-forming gel to ensure healing of dermal wounds [1]. To this end, the gel must limit infections while being non-toxic to the skin cells and not altering normal hemostasis. Initially, we developed two polymers from biocompatible building blocks [1]. These polymers were maintained in a liquid state by segregating the gelation catalysts so that the gel could form to the spatial defect of the wound. We chose to use silver (Ag) as a catalyst as it is widely used as a general antimicrobial agent [13,14] to prevent infections during surgery. Despite this being well documented, the question of whether Ag would be released from the gel to function as an antimicrobial needed to be demonstrated [22,23], as did its relative non-toxicity to the resident skin cells [25-27].
Herein, we demonstrated that the gels were antimicrobial to a range of skin infection agents including yeast, aerobic and anaerobic bacteria. Both compositions of the gel were effective, and primarily microbiocidal in this aspect [14,19]. As Ag must both leave the gel to maintain a relatively aseptic wound bed and remain in the gel to prevent it from becoming a nidus for infection [12], we needed to ascertain its distribution over the 4–7 days of initial wound repair during which infection is most likely to occur. Ag left the PEG gel more efficiently than it left qfrom the lactose gel. Thus, the PEG gel should be preferred in controlling infections in the larger wound bed. However, it is also important to maintain some Ag in the gel, or this can act as a privileged site for an infection nidus, akin to a foreign body [12]. In both cases, at least 20% of the Ag was retained in the gel after 1 week.
The mechanism of Ag dissemination would impact the anti-microbial actions [22,23]. Ag appears to diffuse from the gels as the daily leaching was more extensive than the incremental leaching (compare Figs. 2(b) to (a)). This leaching was not dependent on gel degradation, as this does not occur extensively in the first week [1], while the release of Ag was greatest in the first day, when it would be most needed. As such, the Ag would be able to not only diffuse from the gel, but also back into it if infection set in that matrix.
It was important to determine whether these gels have an adverse impact upon wound healing. While this will require animal studies that await a final gel composition to include local anesthetic, initial assessment would include in vitro assays for cell functions. Neither gels limited proliferation or compromised viability of the two major structural cell types in the skin, dermal fibroblasts and keratinocytes. Interestingly, cell migration, critical for the ingrowth needed to repair spatial defects, was diminished by both gels. As multiple gel components were responsible, we sought to compensate for these defects by adding a migration promoter. Collagen I was chosen for its biological and physiochemical properties [28-30], not least the fact that it occurs naturally in skin. This protein corrected the migration deficit [31-33] when added to PEG gels. As lactose gels were unexpectedly found to interfere with hemostasis, the PEG gel was chosen for further development into a preclinical product.
5. Conclusions
Silver ions served the dual function of catalyzing gelation and providing antimicrobial activity against a broad spectrum of wound contaminating agents. The incorporation of silver within the pre-polymer allows for extended leaching subsequent to gelation of the polymer, with this diffusion presenting the silver akin to current antimicrobial application during surgical interventions. The silver per se presented no adverse impact on cell migration or proliferation. Collagen incorporation into the polymer corrected the negative effects of certain components on cell migration. This multifunctional property of silver allows for a simplified polymer that is biocompatible in vitro while being antimicrobial, thus speeding the process to preclinical and even clinical testing.
Acknowledgements
This work was supported by Grants from the DoD to the National Tissue Engineering Center in Pittsburgh. We are thankful to John Sheaffer and Donald Eisaman for their help in bacteriology work, Bonnie Beiler, Pamala Kaib, Lizz Susanko, Alexus Bacha, Eleanor Zang, and Spiros Giannoutos for their technical assistance and support with spectroscopy experiments, and Barbara Hill for forwarding excess blood samples for hemostasis experiments.
References
- 1.Babu R, Zhang JY, Beckman E, Pasculle AW, Wells A. A polymer-based gel to promote wound healing. Am J Clin Pathol. 2005;124(3):455. [Google Scholar]
- 2.Amadeu TP, Coulomb B, Desmouliere A, Costa AM. Cutaneous wound healing: myofibroblastic differentiation and in vitro models. Int J Low Extrem Wounds. 2003;2(2):60–8. doi: 10.1177/1534734603256155. [DOI] [PubMed] [Google Scholar]
- 3.Klein DG, Fritsch DE, Amin SG. Wound infection following trauma and burn injuries. Crit Care Nurs Clin North Am. 1995;7(4):627–42. [PubMed] [Google Scholar]
- 4.Ovington L. Bacterial toxins and wound healing. Ostomy Wound Manage. 2003;49(7A suppl):8–12. [PubMed] [Google Scholar]
- 5.Guggenbicher JP, Boswald M, Lugauer S, Krall T. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection. 1999;27(Suppl 1):S16–23. doi: 10.1007/BF02561612. [DOI] [PubMed] [Google Scholar]
- 6.Ip M, Lui SL, Poon VK, Lung I, Burd A. Antimicrobial activities of silver dressings: an in vitro comparison. J Med Microbiol. 2006;55(Pt 1):59–63. doi: 10.1099/jmm.0.46124-0. [DOI] [PubMed] [Google Scholar]
- 7.Thomas S, McCubbin P. A comparison of the antimicrobial effects of four silver-containing dressings on three organisms. J Wound Care. 2003;12(3):101–7. doi: 10.12968/jowc.2003.12.3.26477. [DOI] [PubMed] [Google Scholar]
- 8.Holt KB, Bard AJ. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+ Biochemistry. 2005;44(39):13214–23. doi: 10.1021/bi0508542. [DOI] [PubMed] [Google Scholar]
- 9.Bragg PD, Rainnie DJ. The effect of silver ions on the respiratory chain of E. coli. Can J Microbiol. 1974;20(6):883–9. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 10.Darouiche RO. Anti-infective efficacy of silver-coated medical prostheses. Clin Infect Dis. 1999;29(6):1371–7. doi: 10.1086/313561. [DOI] [PubMed] [Google Scholar]
- 11.Chaw KC, Manimaran M, Tay FE. Role of silver ions in destabilization of intermolecular adhesion forces measured by atomic force microscopy in staphylococcus epidermidis biofilms. Antimicrob Agents Chemother. 2005;49(12):4853–9. doi: 10.1128/AAC.49.12.4853-4859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HL, Yuan K, Burgett F, Shyr Y, Syed S. Adherence of oral microorganisms to guided tissue membranes: an in vitro study. J Periodontol. 1994;65(3):211–8. doi: 10.1902/jop.1994.65.3.211. [DOI] [PubMed] [Google Scholar]
- 13.Carr HS, Wlodkowski TJ, Rosenkranz HS. Silver sulfadiazine: in vitro antibacterial activity. Antimicrob Agents Chemother. 1973;4(5):585–7. doi: 10.1128/aac.4.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratton CW, Weeks LS, Aldridge KE. Comparison of Kill-Kinetic studies with agar and broth microdilution methods for determination of antimicrobial activity of selected agents against members of the Bacteroides fragilis group. J Clin Microbiol. 1987;25(4):645–9. doi: 10.1128/jcm.25.4.645-649.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaker JJ, Nazhat SN, Boccaccini AR. Development and character-isation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials. 2004;25(78):1319–29. doi: 10.1016/j.biomaterials.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Waite JH, Benedict CV. Assay of dihydroxyphenylalanine (dopa) in invertebrate structural proteins. Methods Enzymol. 1984;107:397–413. doi: 10.1016/0076-6879(84)07028-2. [DOI] [PubMed] [Google Scholar]
- 17.Arica MY, Bayramoglu G, Arica B, Yalcin E, Ito K, Yagci Y. Novel hydrogel membrane based on copoly(hydroxyethyl methacrylate/p-vinylbenzyl-poly(ethylene oxide)) for biomedical applications: properties and drug release characteristics. Macromol Biosci. 2005;5(10):983–92. doi: 10.1002/mabi.200500091. [DOI] [PubMed] [Google Scholar]
- 18.Andreopoulos FM, Beckman EJ, Russell AJ. Light—induced tailoring of PEG-hydrogel properties. Biomaterials. 1998;19(15):1343–52. doi: 10.1016/s0142-9612(97)00219-6. [DOI] [PubMed] [Google Scholar]
- 19.Gallant-Behm CL, Yin HQ, Liu S, Heggers JP, Langford RE, Olson ME, et al. Comparison of in vitro disc diffusion and time kill-kinetic assays for the evaluation of antimicrobial wound dressing efficacy. Wound Repair Regen. 2005;13(4):412–21. doi: 10.1111/j.1067-1927.2005.130409.x. [DOI] [PubMed] [Google Scholar]
- 20.Wright JB, Lam K, Hansen D, Burrell RE. Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control. 1999;27(4):344–50. doi: 10.1016/s0196-6553(99)70055-6. [DOI] [PubMed] [Google Scholar]
- 21.Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev. 2001;14(2):244–69. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R, Munstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005;26(14):2081–8. doi: 10.1016/j.biomaterials.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 23.Dowsett C. The use of silver-based dressings in wound care. Nurs Stand. 2004;19(7):56–60. doi: 10.7748/ns2004.10.19.7.56.c3736. [DOI] [PubMed] [Google Scholar]
- 24.Otani Y, Tabata Y, Ikada Y. Hemostatic capability of rapidly curable glues from gelatin, poly(l-glutamic acid) and carbodiimide. Biomaterials. 1998;19(22):2091–8. doi: 10.1016/s0142-9612(98)00121-5. [DOI] [PubMed] [Google Scholar]
- 25.Hidalgo E, Dominguez C. Study of cytotoxicity mechanisms of silver nitrate in human dermal fibroblasts. Toxicol Lett. 1998;98(3):169–79. doi: 10.1016/s0378-4274(98)00114-3. [DOI] [PubMed] [Google Scholar]
- 26.Poon VK, Burd A. In vitro cytotoxity of silver: implication for clinical wound care. Burns. 2004;30(2):140–7. doi: 10.1016/j.burns.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 27.Lam PK, Chan ES, Ho WS, Liew CT. In vitro cytotoxicity testing of a nanocrystalline silver dressing (acticoat) on cultured keratinocytes. Br J Biomed Sci. 2004;61(3):125–7. doi: 10.1080/09674845.2004.11732656. [DOI] [PubMed] [Google Scholar]
- 28.Purna SK, Babu M. Collagen based dressings—a review. Burns. 2000;26(1):54–62. doi: 10.1016/s0305-4179(99)00103-5. [DOI] [PubMed] [Google Scholar]
- 29.Patino MG, Neiders ME, Andreana S, Noble B, Cohen RE. Collagen as an implantable material in medicine and dentistry. J Oral Implantol. 2002;28(5):220–5. doi: 10.1563/1548-1336(2002)028<0220:CAAIMI>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Park SN, Kim JK, Suh H. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials. 2004;25(17):3689–98. doi: 10.1016/j.biomaterials.2003.10.072. [DOI] [PubMed] [Google Scholar]
- 31.Grinnell F, B Rocha L, Iucu C, Rhee S, Jiang H. Nested collagen matrices: a new model to study migration of human fibroblast populations in three dimensions. Exp Cell Res. 2006;312(1):86–94. doi: 10.1016/j.yexcr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Helary C, Foucault-Bertaud A, Godeau G, Coulomb B, Guille MM. Fibroblast populated dense collagen matrices: cell migration, cell density and metalloproteinases expression. Biomaterials. 2005;26(13):1533–43. doi: 10.1016/j.biomaterials.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Haga H, Irahara C, Kobayashi R, Nakagaki T, Kawabata K. Collective movement of epithelial cells on a collagen gel substrate. Biophys J. 2005;88(3):2250–6. doi: 10.1529/biophysj.104.047654. [DOI] [PMC free article] [PubMed] [Google Scholar]