Abstract
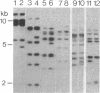
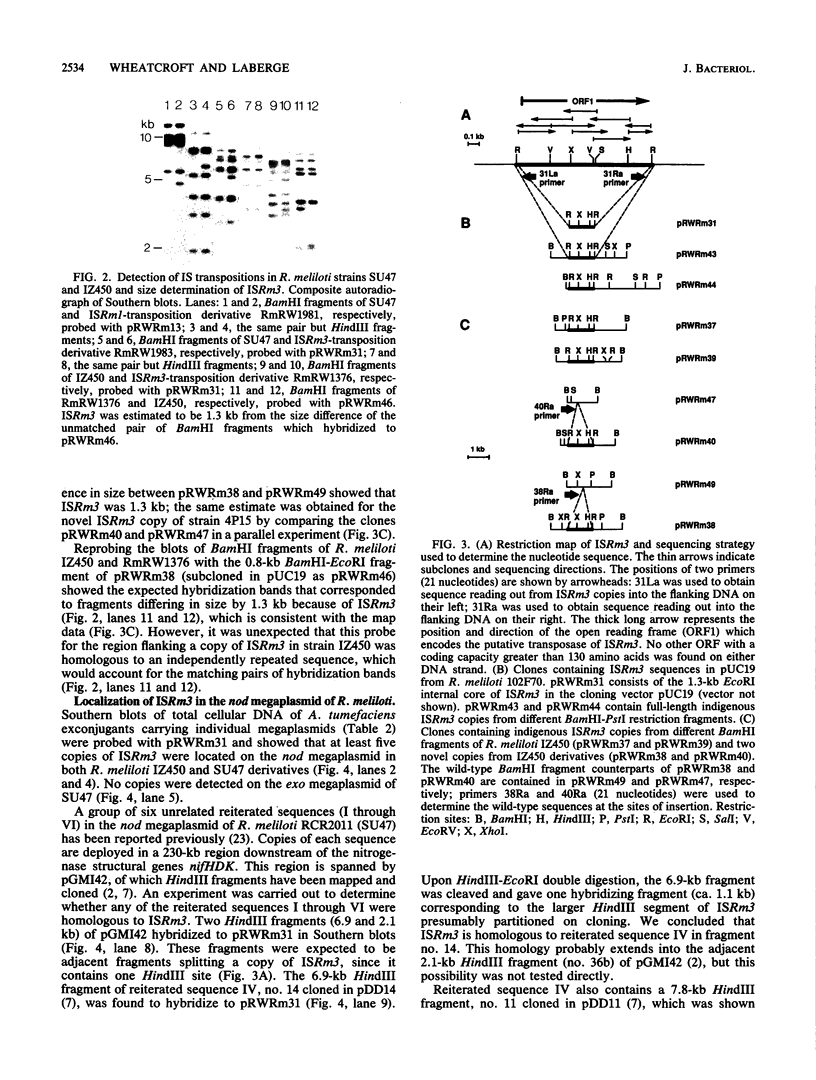
The insertion sequence ISRm3 was discovered simultaneously in different Rhizobium meliloti strains by probing Southern blots of total cellular DNA with 32P-labeled pTA2. This plasmid is indigenous to strain IZ450 and fortuitously contained four copies of ISRm3. By using an internal EcoRI fragment as a specific probe (pRWRm31), homology to ISRm3 was subsequently detected in over 90% of R. meliloti strains tested from different geographical locations around the world. The frequency of stable nonlethal ISRm3 transpositions was estimated to be 4 x 10(-5) per generation per cell in strain SU47 when grown in liquid culture. The entire nucleotide sequence of ISRm3 in R. meliloti 102F70 is 1,298 bp and has 30-bp terminal inverted repeats which are perfectly matched. Analysis of six copies of ISRm3 in two strains showed that a variable number of base pairs (usually eight or nine) were duplicated and formed direct repeats adjacent to the site of insertion. On one DNA strand, ISRm3 contains an open reading frame spanning 93% of its length. Comparison of the putative protein encoded with sequences derived from the EMBL and GenBank databases showed significant similarity between the putative transposases of ISRm3 from R. meliloti, IS256 from Staphylococcus aureus, and IST2 from Thiobacillus ferroxidans. These insertion sequences appear to be distantly related members of a distinct class.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Byrne M. E., Rouch D. A., Skurray R. A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989 Sep 30;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- David M., Domergue O., Pognonec P., Kahn D. Transcription patterns of Rhizobium meliloti symbiotic plasmid pSym: identification of nifA-independent fix genes. J Bacteriol. 1987 May;169(5):2239–2244. doi: 10.1128/jb.169.5.2239-2244.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusha I., Kovalenko S., Banfalvi Z., Kondorosi A. Rhizobium meliloti insertion element ISRm2 and its use for identification of the fixX gene. J Bacteriol. 1987 Apr;169(4):1403–1409. doi: 10.1128/jb.169.4.1403-1409.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardly B. D., Materon L. A., Smith N. H., Johnson D. A., Rumbaugh M. D., Selander R. K. Genetic structure of natural populations of the nitrogen-fixing bacterium Rhizobium meliloti. Appl Environ Microbiol. 1990 Jan;56(1):187–194. doi: 10.1128/aem.56.1.187-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Kunkel B., De Vos G. F., Signer E. R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986 Jul;167(1):66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V. N., Klee H. J., Nester E. W. Units of genetic expression in the virulence region of a plant tumor-inducing plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1982;188(3):418–424. doi: 10.1007/BF00330043. [DOI] [PubMed] [Google Scholar]
- Laberge S., Gagnon Y., Bordeleau L. M., Lapointe J. Cloning and sequencing of the gltX gene, encoding the glutamyl-tRNA synthetase of Rhizobium meliloti A2. J Bacteriol. 1989 Jul;171(7):3926–3932. doi: 10.1128/jb.171.7.3926-3932.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renalier M. H., Batut J., Ghai J., Terzaghi B., Gherardi M., David M., Garnerone A. M., Vasse J., Truchet G., Huguet T. A new symbiotic cluster on the pSym megaplasmid of Rhizobium meliloti 2011 carries a functional fix gene repeat and a nod locus. J Bacteriol. 1987 May;169(5):2231–2238. doi: 10.1128/jb.169.5.2231-2238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Long S. R., Meade H. M., van den Bos R. C., Ausubel F. M. ISRm1: A Rhizobium meliloti insertion sequence that transposes preferentially into nitrogen fixation genes. J Mol Appl Genet. 1982;1(5):405–418. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatcroft R., Watson R. J. A Positive Strain Identification Method for Rhizobium meliloti. Appl Environ Microbiol. 1988 Feb;54(2):574–576. doi: 10.1128/aem.54.2.574-576.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatcroft R., Watson R. J. Distribution of insertion sequence ISRm1 in Rhizobium meliloti and other gram-negative bacteria. J Gen Microbiol. 1988 Jan;134(1):113–121. doi: 10.1099/00221287-134-1-113. [DOI] [PubMed] [Google Scholar]
- Wheatcroft R., Williams P. A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981 Jun;124(2):433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yates J. R., Cunningham R. P., Holmes D. S. IST2: an insertion sequence from Thiobacillus ferrooxidans. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7284–7287. doi: 10.1073/pnas.85.19.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]