Abstract
The involvement of p53 in regulating diverse cellular processes dictates that it must respond to multiple signaling mechanisms, thus coordinating the response to various “stress conditions.” Genotoxic stress has served as a paradigm to dissect the transactivation-dependent branch of the pathway by which p53 can induce growth arrest. Alternate mechanisms have been invoked to explain transactivation-independent effects of p53, especially in the context of apoptosis. We have identified a p53-dependent pathway initiated by the gas1 product, a plasma membrane protein highly expressed during G0, which activates a transactivation-independent p53 growth arrest function. Through a detailed deletional analysis and site-specific mutagenesis of p53 we show that the Gas1-dependent signal transduction relies on a proline-rich region (amino acids 63–85) of murine p53. In vivo competition experiments using combinations of such mutants implicate this functional domain of p53 as a docking site in the transmission of antiproliferative signals.
Wild-type (wt) p53 can mediate a variety of antiproliferative effects, such as induction of G1 and G2 arrest, activation of apoptotic cell death and suppression of oncogene-mediated transformation (1–3). Four structural and functional domains have been identified in the p53 protein and some of them are required and sufficient for specific functions (4). Genotoxic stress as induced by various types of chemical and physical DNA damaging agents, has been the most studied and used inducer of p53 functions. Accumulating evidence indicates that p53 can respond to other stress stimuli that do not appear to induce, at least directly, DNA damage. Hypoxic conditions (5), heat shock (6), calcium phosphate treatment (7), or ribonucleotide depletion (8) have all been reported to activate p53 functions. A common event in the response to such stress stimuli is that the cellular levels of p53 dramatically rise, mainly due to posttranslational stabilization of the protein (3, 9, 10). In normal fibroblasts the commonly observed consequence of enhanced p53 levels, as induced by DNA damaging agents, is G1 arrest that presumably allows repair of the DNA damage before entry into S phase (4, 11). The sequence-specific transactivation function of p53 has been shown to be instrumental in establishing G1 arrest and among the genes induced by p53 the most important candidate to mediate cell cycle block is p21/waf1 (12–14). Increased levels of p21/waf1 have been shown to interfere with the cell cycle machinery through the combined inhibition of the cyclin/cdk kinases and the replicative functions of the DNA polymerase δ-associated factor PCNA (15, 16). However, p21/waf1 deficient mice (17, 18) retain p53-dependent G1 arrest function, albeit compromised relative to the normal controls. These results suggest the existence of yet another p53-dependent G1 arrest pathway that either requires transactivation of other genes or else operates through a different function and perhaps a different domain of p53.
It has been widely described that p53 is able to directly interact with a multitude of cellular and viral proteins (4). Therefore, it may well be possible that an alternative domain of p53 is responsible for a specific interaction with a yet uncharacterized cellular protein, which mediates the growth suppressing function of p53. Involvement of such an alternative function has particularly been implicated in the case of p53-dependent apoptosis (2). In fact, although in some cases (19, 20) p53 mutants unable to transactivate are also completely deficient in apoptosis, other reports indicate that the sequence-specific transactivation function is either dispensable or at least not fully sufficient (21–23).
Nontransformed fibroblasts in culture respond to growth factor deprivation by exiting the cell cycle and entering a reversible growth arrest or G0. This state is marked by the increased expression of the gas (growth arrest specific) genes that become rapidly downregulated upon readdition of growth factors. The function of gas genes has been linked to the regulation of apoptosis at various levels (24–26) and in the case of Gas1 to growth suppression (27, 28). When Gas1, a plasma membrane glycoprotein, is ectopically expressed it blocks the G0 to S transition of quiescent fibroblasts and the cell cycle progression in various types of asynchronously growing cells (27, 28). We have demonstrated that this effect of Gas1 requires wt p53 but is independent of its transactivation function (29). The aim of this work was therefore to elucidate the p53 domains that are essential for mediating Gas1 function to gain new insights into how a membrane associated protein could exert its suppressive function via p53.
MATERIALS AND METHODS
Plasmids.
wt murine p53 cDNA was excised from pMSVcL vector (30) and subcloned in pGDSV7 under the control of simian virus 40 early promoter (31) to generate pGDSV7-mp53. Numbering of murine p53 amino acids is done according to Soussi et al. (32), assuming that the second in-frame ATG is used as initiation codon. Deletion Δ(8–63) was generated by digestion of pGDSV7-mp53 with EcoRV–SacI, elimination of protruding ends with T4 DNA polymerase and re-ligation. Deletion Δ(8–106) was created by insertion of an oligonucleotide adaptor between the EcoRV and PstI sites in mp53. Deletion Δ(8–85) was produced by inserting a PCR amplified fragment corresponding to amino acids 86–106 in the EcoRV site of the Δ(8–106) construct. Deletion M154 was created by fusion of the two NcoI sites corresponding to Met-1 and Met-154 by NcoI digestion, removal of the fragment and religation. Deletion Δ(8–63)dl330 was generated by assembling the truncated C terminus from pCMVdl330 (33) as a KpnI–XbaI fragment in the Δ(8–63) construct. Plasmid p53wt-5980 was generated by PCR-based directed mutagenesis, on the background of plasmid pCMVp53wt (34). Construct Δ(8–63)/5980 was created by replacing the PstI fragment spanning amino acid 64 to 109 in the Δ(8–63) mutant with the corresponding fragment derived from p53wt-5980 and clone Δ(8–63)dl330/5980 was generated by assembling the N terminus from Δ(8–63)/5980 as an XhoI–KpnI fragment on the Δ(8–63)dl330 construct. All constructs were sequenced to confirm the introduced changes using an automated DNA sequencer (ALF–Pharmacia).
In Vitro Translation and Electrophoretic Mobility-Shift Assay.
The expression vectors containing the mp53 deletion constructs were directly used for in vitro transcription/translation, using the TNT T7 Coupled Reticulocyte Lysate system (Promega) according to the manufacturer’s instructions. DNA-binding reactions were performed in a volume of 15 μl: in vitro translated proteins were incubated with ≈105 cpm of 32P-labeled, annealed p53CON oligonucleotide (5′-GGACATGCCCGGGCATGTCC-3′) (35), in binding buffer (10 mM Tris⋅HCl, pH 8/100 mM NaCl/1 mM EDTA/8 mM spermidine/1 mM DTT/5% glycerol/0.2 μg/μl BSA/100 ng salmon sperm DNA) at room temperature for 20 min. All the reactions contained 2 μl PAb421 hybridoma supernatant. Complexes were loaded on 4.5% nondenaturing polyacrylamide gel and electrophoresed at 4°C. A sheet of plastic foil was placed between dried gel and autoradiographic film to cut out the signal from [35S]methionine. Specificity of the binding was confirmed by competition with excess cold p53CON or p53MUT (5′-GGACAGTCGGCCGACTGTCC-3′) oligonucleotides.
Microinjection and Immunofluorescence.
Cells cultured in DMEM containing 10% fetal calf serum were grown on coverslips (5 × 103 cells per cm2) in 35-mm diameter Petri dishes. After 24 hr of incubation at 37°C in 5% CO2 atmosphere, cells were microinjected by the Automated Injection system (Zeiss) as described (27). For expression analysis they were incubated for 12 hr and then processed for immunofluorescence. For growth inhibition assay, after injection cells were left for 24 hr in DMEM supplemented with 10% fetal calf serum. DNA synthesis assay was performed by adding 50 μM BrdUrd to the culture medium 6 hr before fixation. Cells were fixed and processed for immunofluorescence as described (27). mp53 was revealed by using the PAb240 antibody followed by goat anti-mouse IgG1-fluorescien isothiocyanate (Southern Biotechnology Associates); BrdUrd was revealed by mAb anti-BrdUrd (Amersham) followed by goat anti-mouse IgG2a-rhodamine isothiocyanate (Southern Biotechnology Associates). p21/waf1 was revealed by the affinity purified antibody described in Del Sal et al. (29) followed by goat anti-rabbit RITC (Southern Biotechnology Associates).
RESULTS
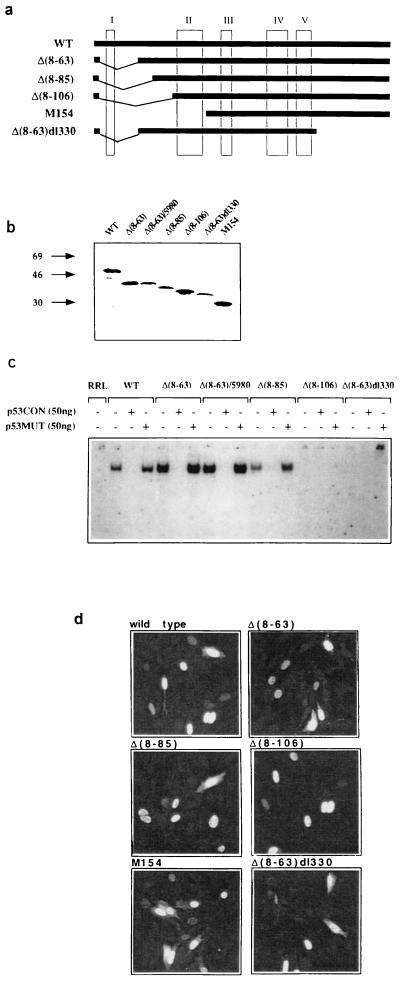
To identify the p53 domains that are essential for mediating the Gas1 induced growth arrest, a series of deletion mutants was generated from wt murine p53 (see Materials and Methods) as shown in Fig. 1a. All mutants, except Met-154, are internal deletions in which the original translation start site has been conserved. Analysis of the respective in vitro translation products by SDS/PAGE confirmed their expected size (Fig. 1b). We next tested the ability of the p53 deletion mutant products to form gel-stable complexes with the p53CON oligonucleotide by a mobility retardation assay (electrophoretic mobility-shift assay) as shown in Fig. 1c. Mutants with small N-terminal deletions [Δ(8–63) and Δ(8–85)] specifically bind to the consensus sequence similarly to wt p53, and this binding is competed by addition of excess specific oligo. Larger N-terminal deletion mutants [Δ(8–106) and Met-154] are severely compromised in DNA binding activity, as is also noticed for the amino/carboxyl double deletion lacking the oligomerization domain [Δ(8–63)dl330].
Figure 1.
Expression, sequence specific DNA binding activity, and nuclear localization of murine p53 deletion mutants. (a) Schematic representation of the p53 mutants used. Numbering of murine p53 amino acids is done according to ref. 32. (b) In vitro translated murine p53 mutants were analyzed by SDS/PAGE to confirm the expected apparent molecular weights. The structure of deletion Δ(8–63)/5980 is described in Fig. 3. (c) In vitro translated proteins were tested for sequence specific DNA binding by electrophoretic mobility shift assay with the p53CON oligonucleotide as a probe. Specificity of the binding was confirmed by competition with excess cold p53CON or p53MUT oligonucleotides. (d) 100 ng/μl of each pGDSV7 expression plasmid (31) containing the different p53 deletion mutants, was microinjected into the nuclei of BALB/c (10)1 cells (36). p53 protein expression was analyzed 12 hr later by indirect immunofluorescence using PAb240. Images were obtained with a LSM 450 confocal microscope (Zeiss).
To evaluate the subcellular localization and expression level of the p53 deletion mutants, each expression vector was microinjected in BALB/c (10)1 (p53 null) fibroblasts (36). The mutant proteins accumulated into the nucleus similarly to wt p53 as assessed by immunofluorescence (Fig. 1d).
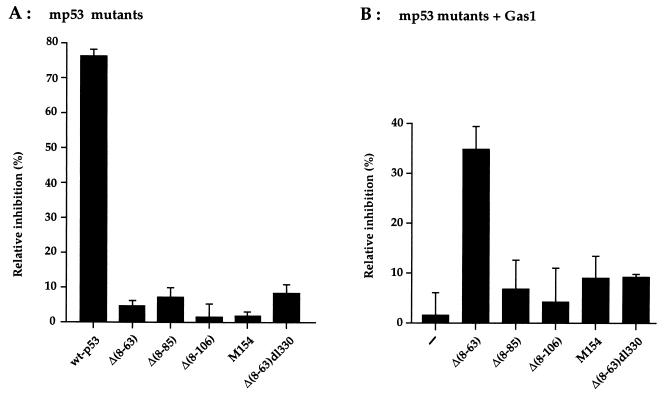
Next, the mutants were analyzed for their ability to induce growth arrest in BALB/c (10)1 fibroblasts. Ectopic expression of the various mutants was performed by microinjection, and its effect on cell cycle was evaluated by measuring the relative level of BrdUrd incorporation (S-phase). All the p53 mutants were defective in inhibiting DNA synthesis, whereas wt p53 exhibited a strong inhibitory effect (Fig. 2A). As previously shown (29), expression of Gas1 alone is also unable to inhibit DNA synthesis in these cells (Fig. 2B). Next, we evaluated if any of the described p53 mutants was able to reconstitute the Gas1-dependent growth arrest pathway; this was done by coexpressing Gas1 with the various deletion mutants (Fig. 2B). Only mutant Δ(8–63) was able to cooperate with Gas1 to inhibit DNA synthesis to a similar extent as obtained when Gas1 is overexpressed in cells harboring endogenous wt p53 (27, 28). The Δ(8–63) p53 mutant lacks the entire transcriptional activation domain indicating that not only the transactivation function but the structural domain itself is dispensable for mediating Gas1 induced growth arrest. From Fig. 2B it can also be deduced that integrity of the DNA binding domain is critical for cooperation with Gas1. In fact, deletions affecting DNA binding activity [Δ(8–106) and Met-154] are also defective in the ability to reconstitute Gas1 induced growth suppression (Fig. 2B). In addition the double deletion Δ(8–63)dl330, also lacking the oligomerization domain, loses the ability to cooperate with Gas1, in line with the observation that efficient DNA binding requires the assembly of p53 into homotetramers (37–39). This is in line with our previous results showing that point mutations affecting the DNA binding domain, Val-135 (at 37°C) and KH215, are not able to cooperate with Gas1 (29).
Figure 2.
Growth arrest analysis of the p53 deletion mutants in BALB/c (10)1 fibroblasts. (A) Relative inhibition of BrdUrd incorporation by expression of the various p53 mutants. (B) Relative inhibition of BrdUrd incorporation after coexpression of the various p53 mutants in combination with Gas1. pGDVS7 expression vector (100 ng/μl of each) was microinjected into the nuclei of growing BALB/c (10)1 fibroblasts. When Gas1 was expressed together with the p53 deletion mutants, 50 ng/μl of each plasmid was used. Eighteen hours after injection 50 μM BrdUrd was added to the culture medium to monitor S phase. After 6 hr cells were fixed and processed for immunofluorescence as described in Materials and Methods. The percentage of relative inhibition of DNA synthesis in the injected cells was calculated by the following formula: % relative inhibition = [% BrdUrd-positive cells (uninjected) − % of BrdUrd-positive cells (p53/Gas1-positive)/% of BrdUrd-positive cells (uninjected)]. The mean of three independent experiments with at least 300 overexpressing cells scored is shown.
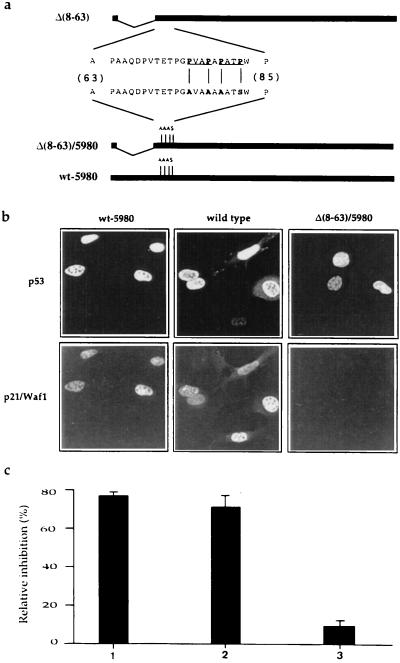
Most significantly, we could demonstrate that sequence specific DNA binding activity per se is not sufficient to reconstitute the Gas1 growth suppression pathway. In fact, in stark contrast to the Δ(8–63) deletion, mutant Δ(8–85) is completely defective in reconstituting the Gas1 induced growth arrest (Fig. 2B). Similarly to Δ(8–63), mutant Δ(8–85) retains both wt conformation and sequence-specific DNA binding (Fig. 1c). This last observation strongly suggested that the region between amino acids 63–85 could represent a new functional domain of p53 that is critical in mediating the Gas1-dependent growth arrest. The region of murine p53 between residues 63–85 contains several prolines (Fig. 3a). In particular, the PXXP motif has been described as a docking site for interaction with SH3 domains (40, 41). The corresponding human p53 sequence contains five such motifs while murine p53 contains only two, the first one being conserved between human and mouse. To analyze whether the prolines contained in this region could be responsible for cooperation of p53 with Gas1, point mutations were generated by replacing Pro-76, -79, and -81 with alanines, and Pro-84 with serine. The mutations were introduced into both full-length p53 (p53wt-5980) and N-terminal truncated p53 (Δ(8–63)/5980) (Fig. 3a). Both mutant proteins localized to the nuclei of microinjected BALB/c (10)1 cells (Fig. 3b). Since Δ(8–63)/5980 retains sequence specific DNA binding (Fig. 1c), we evaluated the ability of the mutants to exert sequence specific transactivation by measuring the induction of p21/Waf1 protein in BALB/c (10)1 cells. The p53wt-5980 mutant, retaining the transactivation domain, induced efficient expression of endogenous p21/Waf1, while the transactivation deficient Δ(8–63)/5980 mutant completely lacked such activity (Fig. 3b). As expected from this observation, the growth inhibitory activity of p53wt-5980 was comparable to that of wt p53, whereas Δ(8–63)/5980 was found to lack such activity (Fig. 3c).
Figure 3.
Structure, p21/Waf1 induction and growth arrest function analysis of proline-rich region mutants. (a) Amino acid sequence of the proline-rich region in mouse p53. The PXXP motifs are underlined; the substituted amino acids in the mutated version are indicated in bold letters. Clone p53wt-5980 codes for a murine p53 bearing four point mutations that substitute Pro-76, -79, and -81 with alanines, and Pro-84 with serine. (b) Proline substitution in the p53 sequence does not interfere with its ability to transactivate endogenous p21/Waf1 as assayed by the induction of the corresponding protein product. Expression plasmids were microinjected in BALB/c (10)1 cells. Six hours later cells were fixed and analyzed for expression of the p53 protein and of endogenous p21/Waf1 by immunofluorescence. p21/Waf1 was detected by anti-peptide antibody as described (29). Wt p53 and Δ(8–63)/5980 were used as positive and negative controls, respectively. (c) Analysis of the growth arrest function of p53 wt-5980 (1), wt p53 (2), and Δ(8–3)/5980 (3). BrdUrd incorporation was monitored as described in legend to Fig. 2.
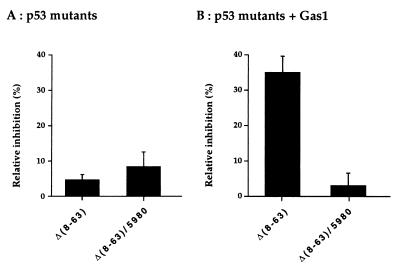
Next, we analyzed the ability of the proline mutated Δ(8–63)/5980 to reconstitute the Gas1-dependent growth arrest function. Most remarkably, this mutant totally failed to cooperate with Gas1 to induce growth arrest, in stark contrast to the proline-containing Δ(8–63)mutant (Fig. 4). This strongly suggests that the proline-rich region defines a domain within p53 required to transduce a signal from the plasma membrane, where Gas1 is localized, eventually resulting in growth suppression.
Figure 4.
Mutation of Pro-76, -79, -81, and -84 within deletion mutant p53 Δ(8–63) affects the ability to reconstitute the Gas1-dependent growth arrest function. (A) Relative inhibition of BrdUrd incorporation by expression of Δ(8–63) or Δ(8–63)/5980 p53 mutants. (B) Relative inhibition of BrdUrd incorporation by co-expression of the same p53 mutants in combination with Gas1. Microinjection experiments were performed as described in legend to Fig. 2. The mean of three independent experiments with at least 300 overexpressing cells scored are shown.
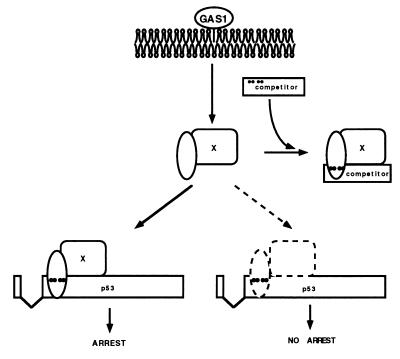
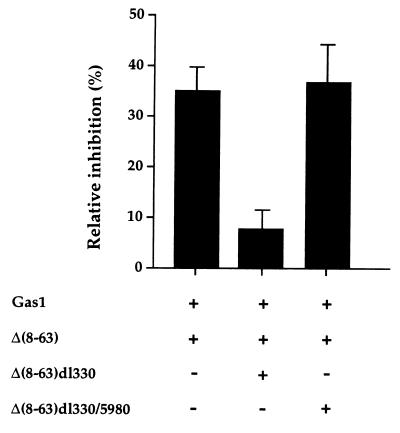
We next wished to gain insight into the molecular mode of action of this domain. Given its evolutionary conservation, a reasonable hypothesis is that this domain may mediate growth suppression via Gas1-regulated interaction with a cellular protein containing an SH3 domain. We therefore reasoned that a truncated form of p53, retaining the proline-rich domain and the DNA binding domain but lacking both transactivation and oligomerization domains [Δ(8–63)dl330], might behave as an efficient competitor for the postulated interaction and prevent its biological consequences (Fig. 5). On the other hand such competition should not be exerted by the same truncated form of p53 missing the critical prolines [Δ(8–63)dl330/5980]. To test this prediction, we performed a triple co-expression experiment where either the Δ(8–63)dl330 or Δ(8–63)dl330/5980 truncation mutants were microinjected together with Gas1 and the Δ(8–63) mutant into BALB/c (10)1 cells. As seen in Fig. 6, the truncated Δ(8–63)dl330 mutant indeed interfered efficiently with growth arrest induced by the complementing co-expression of Gas1 and Δ(8–63), but this interference was abolished by mutation of the critical prolines, as in Δ(8–63)dl330/5980. Experiments were scored either for Gas1 or p53 expression relative to BrdUrd incorporation giving similar results. The “proline specificity” of the observed competition strongly supports the notion that a cellular protein(s) interacting with the proline-rich motif of p53 is responsible for mediating Gas1-induced growth arrest.
Figure 5.
Model for Gas1 signaling via the proline-rich domain of p53. The proline-rich domain of p53 (dots indicate the four relevant prolines) may signal for growth suppression via contacting the SH3 domain of a cellular protein (X). Such interaction should be modulated by signals departing from the plasma membrane where Gas1 is localized. A p53 mutant lacking the tetramerization domain (competitor) could interfere with growth suppression by sequestering the cognate SH3-containing cellular protein.
Figure 6.
Triple co-expression of Gas1 plus Δ(8–63) and either Δ(8–63)dl330 or Δ(8–63)dl330/5980 deletion mutants in BALB/c (10)1 cells. Δ(8–63)dl330 mutant, that retains the proline-rich domain but lacks the transactivation and oligomerization domains, behaves as an efficient competitor for the Gas1/Δ(8–63) inhibitory effect. Competition is not observed with the corresponding proline-substituted mutant Δ(8–63)dl330/5980. Microinjection experiments were performed as described in legend to Fig. 2. pGDSV7-gas1 (50 ng/μl) plus 30 ng/μl of pGDSV7-Δ(8–63), and 30 ng/μl of either pGDSV7-Δ(8–63)dl330 or pGDSV7-Δ(8–63)dl330/5980 were microinjected into BALB/c (10)1 cells. Experiments were scored either for Gas1 or p53 expression relative to BrdUrd incorporation giving similar results. Mean ± SD.
DISCUSSION
The p53 tumor suppressor protein is capable of responding to multiple cellular stress signals and, depending on the type of signal and the cellular context, the integrated p53 response can result in transient cell cycle arrest compatible with survival or apoptosis. A large body of evidence indicates the important function of p53 as a transcription factor, mediating such effects via the activation of a specific genetic program. Such activity, however, can sometimes be dispensable for the apoptotic p53 pathway (2). Therefore, given the complexity of stimuli that p53 is called to integrate into growth arrest/survival (42) or apoptosis, it is conceivable that, in addition to the transactivation function, different domains of the p53 protein directly modulate the transmission of specific signals by interaction with other cellular proteins. The availability of a biological assay that allows one to discriminate transactivation-independent p53 functions should therefore be instrumental in identifying such new domains and the mechanisms involved. In this context we have shown that ectopically expressed Gas1 exerts growth suppression activity in various cells types (27, 28) through a p53-mediated mechanism that is transactivation- independent (29). This represented a clear case where the transactivation function of p53 was dispensable for the p53-dependent biological response. We therefore wished to identify the region/domain of p53 that is specifically required for mediating the growth suppressing Gas1 signal. Analysis of various p53 deletion mutants established rigorously that the transactivation domain is structurally dispensable for Gas1 complementation in p53 null cells. Full DNA binding function of p53 was shown to be required but not sufficient for complementation. Mutants Δ(8–63) and Δ(8–85) were particularly instructive since only the former was able to complement Gas1. Both mutants retaining full DNA binding ability, the only difference between the two mutants is the presence of the proline-rich region contained between amino acids 63–85. Deletion of the corresponding region (amino acids 61–94) in human p53 impairs growth suppression of tumor cell growth in culture, as determined by colony formation assays (43). Such a proline-rich region therefore defines a new domain of p53, involved in the transactivation-independent transduction of the Gas1 growth arrest signal. Within this domain we were able to identify a critical motif, encompassing Pro-76–84 and representing a potential binding site for SH3 domains. Mutant Δ(8–63), containing substituted prolines at positions 76, 79, 81, and 84, was defective in complementing the Gas1-dependent growth arrest. Because of the well documented roles that PXXP motifs play in the formation of signaling complexes through the binding of cognate SH3 domains, we hypothesized that this motif could similarly act via the binding of a putative cellular protein involved in the transmission of the growth arrest signal. Our data suggest that, in addition to the PXXP motif, this interaction requires wt conformation of the nearby DNA binding domain. It is not uncommon that interactions between domains such as SH3 and docking sites are also influenced by the conformation of the surrounding domains (41). Interaction with a cellular protein, requiring both the PXXP and the DNA binding domain of p53, may represent a final regulatory event in the Gas1-dependent signal transduction cascade initiated at the plasma membrane (Fig. 5). The competition experiments support this working model. The truncated mutant containing the proline-rich domain and the DNA binding core was indeed able to block the complementation in the Gas1 growth arrest, but this was not observed using the proline mutant. A candidate protein, known to interact with p53 and containing an SH3 domain, could be 53BP2 (44, 45). The recently reported crystal structure of the complex between p53 and 53BP2 (46) demonstrates, however, that the SH3 domain of 53BP2 interacts with the L3 loop of p53’s central core domain (amino acid241–248) in a manner distinct from that of previously characterized SH3 complexes. This leaves open the identity of the protein responsible for the interaction and the activities of the proline-rich domain described here. Altogether our results identify a novel functional domain within p53, which highlights the ability of p53 to directly participate in the transmission of antiproliferative signals in a manner not necessarily requiring its activity as a transcription factor. Such a domain likely represents a target site for interaction with elements of signal transduction pathways that transduce stress signals. In this scenario Gas1 might represent one element, located at the plasma membrane, that is involved in the transduction of a critical signal during G0 when Gas1 is maximally expressed. Identification of the cellular proteins capable of specifically interacting with the identified proline domain of p53 will greatly increase our understanding of this pathway and of p53 function in general.
Acknowledgments
This work was generously supported by funding from Associazione Italiana Ricerca sul Cancro and Consiglio Nazionale delle Richerche–Applicazioni Cliniche della Ricerche Oncologica to C.S., Progetto Strategico Consiglio Nazionale delle Richerche to G.D.S., and by U.S. Public Health Service Grant RO1 CA40099 from National Cancer Institute to M.O.
ABBREVIATION
- wt
wild type
References
- 1.Levine A J. Annu Rev Biochem. 1993;63:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- 2.Bates S, Vousden K H. Curr Opin Gen Dev. 1996;6:12–19. doi: 10.1016/s0959-437x(96)90004-0. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb T M, Oren M. Biochem Biophys Acta Rev Cancer. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- 4.Ko L J, Prives C. Genes Dev. 1996;9:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 5.Graeber T G, Peterson J F, Tsai M, Monica K, Fornace A J, Jr, Giaccia A J. Mol Cell Biol. 1994;14:6264–6277. doi: 10.1128/mcb.14.9.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohnishi T, Wang X, Ohnishi K, Matsumoto H, Takahashi A. J Biol Chem. 1996;271:14510–14513. doi: 10.1074/jbc.271.24.14510. [DOI] [PubMed] [Google Scholar]
- 7.Renzing J, Lane D P. Oncogene. 1995;10:1865–1868. [PubMed] [Google Scholar]
- 8.Linke S P, Clarkin K C, Di Leonardo A, Tsou A, Whal G M. Genes Dev. 1996;10:934–947. doi: 10.1101/gad.10.8.934. [DOI] [PubMed] [Google Scholar]
- 9.Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R W. Cancer Res. 1991;51:6304–6311. [PubMed] [Google Scholar]
- 10.Chowdary D R, Dermody J J, Jha K K, Oze H L. Mol Cell Biol. 1994;14:1997–2003. doi: 10.1128/mcb.14.3.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Leonardo A, Linke S P, Clarkin K, Wahl M. Genes Dev. 1994;8:2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- 12.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parson R, Trent J M, Lin D, Mercer E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 13.Harper W J, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 14.Xiong Y, Hannon G J, Zhang H, Casso D, Beach D. Nature (London) 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Jackson P K, Kirshner M W, Dutta A. Nature (London) 1995;374:368–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y, Hurvitz J, Massague J. Nature (London) 1995;375:159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 17.Deng C, Zang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 18.Brugarolas J, Chandrasekaran C, Gordon J I, Beach D, Jacks T, Hannon T. Nature (London) 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 19.Sabbatini P, Lin J, Levine A J, White E. Genes Dev. 1995;9:2184–2192. doi: 10.1101/gad.9.17.2184. [DOI] [PubMed] [Google Scholar]
- 20.Attardi L D, Lowe S W, Brugarolas J, Jacks T. EMBO J. 1996;15:3693–3701. [PMC free article] [PubMed] [Google Scholar]
- 21.Caelles C, Helmberg A, Karin M. Nature (London) 1994;370:220–223. doi: 10.1038/370220a0. [DOI] [PubMed] [Google Scholar]
- 22.Haupt Y, Rowan S, Shaulian E, Vousden K H, Oren M. Genes Dev. 1995;9:2170–2183. doi: 10.1101/gad.9.17.2170. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Vermeulen W, Coursen J D, Gibson M, Lupold S E, Forrester K, Xu G, Elmore L, Yeh H, Hoeijmakers J H J, Harris C. Genes Dev. 1996;10:1219–1232. doi: 10.1101/gad.10.10.1219. [DOI] [PubMed] [Google Scholar]
- 24.Brancolini C, Benedetti M, Schneider C. EMBO J. 1995;14:5179–5190. doi: 10.1002/j.1460-2075.1995.tb00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabretti E, Edomi P, Brancolini C, Schneider C. Genes Dev. 1995;9:1846–1856. doi: 10.1101/gad.9.15.1846. [DOI] [PubMed] [Google Scholar]
- 26.Goruppi S, Ruaro E, Schneider C. Oncogene. 1996;12:471–480. [PubMed] [Google Scholar]
- 27.Del Sal G, Ruaro M E, Philipson L, Schneider C. Cell. 1992;70:595–607. doi: 10.1016/0092-8674(92)90429-g. [DOI] [PubMed] [Google Scholar]
- 28.Del Sal G, Collavin L, Ruaro M E, Edomi P, Saccone E, Della Valle G, Schneider C. Proc Natl Acad Sci USA. 1994;91:1848–1852. doi: 10.1073/pnas.91.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Sal G, Ruaro M E, Utrera R, Cole N C, Levine A J, Schneider C. Mol Cell Biol. 1995;15:7152–7160. doi: 10.1128/mcb.15.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finlay C A, Hinds P W, Tan T H, Eliyahu D, Oren M, Levine A J. Mol Cell Biol. 1988;8:531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Sal G, Manfioletti G, Gustincich S, Ruaro M E, Schneider C. BioTechniques. 1994;16:134–138. [PubMed] [Google Scholar]
- 32.Soussi T, Caron de Formentel C, May P. Oncogene. 1990;5:945–952. [PubMed] [Google Scholar]
- 33.Shaulian E, Haviv I, Shaul Y, Oren M. Oncogene. 1995;10:671–680. [PubMed] [Google Scholar]
- 34.Eliyahu D, Michalowitz D, Eliyahu S, Pinhasi-Kimhi O, Oren M. Proc Natl Acad Sci USA. 1989;86:8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funk W D, Pak D T, Karas R H, Wright W E, Shay J W. Mol Cell Biol. 1992;12:2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey D M, Levine A J. Genes Dev. 1991;5:227–234. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 37.Tarunina M, Jenkins J. Oncogene. 1993;8:3165–3173. [PubMed] [Google Scholar]
- 38.Shaulian E, Zaubermann A, Milner J, Davies E A, Oren M. EMBO J. 1993;12:2789–2795. doi: 10.1002/j.1460-2075.1993.tb05940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Schwedes J F, Parks D, Mann K, Tegtmeyer P. Mol Cell Biol. 1995;15:2157–2165. doi: 10.1128/mcb.15.4.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexandropoulos K, Cheng G, Baltimore D. Proc Natl Acad Sci USA. 1995;92:3110–3114. doi: 10.1073/pnas.92.8.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee C-H, Saksela K, Mirza U A, Chait B T, Kuriyan J. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 42.Lassus P, Ferlin M, Piette J, Hibner U. EMBO J. 1996;15:4566–4573. [PMC free article] [PubMed] [Google Scholar]
- 43.Walker K K, Levine A J. Proc Natl Acad Sci USA. 1996;93:15335–15340. doi: 10.1073/pnas.93.26.15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwabuchi K, Bartel P L, Li B, Maraccino R, Fields S. Proc Natl Acad Sci USA. 1994;91:6098–6102. doi: 10.1073/pnas.91.13.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thukral S K, Blain G C, Chang K H, Fields S. Mol Cell Biol. 1994;14:8315–8321. doi: 10.1128/mcb.14.12.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorina S, Pavletich N P. Science. 1996;274:1001–1005. doi: 10.1126/science.274.5289.1001. [DOI] [PubMed] [Google Scholar]