Abstract
Generalized low bone mass and osteopenia in both axial and peripheral skeleton in adolescent idiopathic scoliosis (AIS) have been reported in literature. However, the exact mechanisms and causes of the bone loss in AIS are not identified yet. Therefore, this study examined the relationship between serum concentration of soluble receptor activator of nuclear factor-κB ligand (RANKL), serum level of osteoprotegerin (OPG) and bone mass in 72 patients with AIS and compared to those of 64 age- and gender-matched healthy controls. The mean lumbar spinal bone mineral density (LSBMD) and femoral neck BMD (FNBMD) in patients with AIS were decreased compared with that in control individuals, respectively (P = 0.0029 and P = 0.0192, respectively). The mean RANKL and RANKL to OPG ratio in patients with AIS were increased compared with that in control subjects, respectively (P = 0.0004 and P = 0.0032, respectively). The RANKL and RANKL to OPG ratios were negatively correlated to the LSBMD and serum OPG levels in both groups. Serum OPG levels were positively correlated to the LSBMD and FNBMD in both groups. These findings mean that the imbalance and the disturbed interaction of RANKL and OPG may be an important cause and pathogenesis in reduced BMD in AIS.
Keywords: Adolescent idiopathic scoliosis, Bone mineral density, RANKL, OPG
Introduction
Adolescent idiopathic scoliosis (AIS) is a complex three-dimensional deformity of the spine occurring mostly in girls between 10 and 14 years old. The etiology and pathogenesis of idiopathic scoliosis remain unclear despite the large number of studies performed. The cause of scoliosis is believed to be multifactorial because of the association between the development of scoliosis and growth, hormonal secretion, gravity, etc. [1, 12, 13, 16, 20, 22, 23, 28, 29, 31]. However, none of these parameters have been conclusively shown to have a causative role in AIS development.
Association of osteopenia with idiopathic scoliosis was first reported by Burner and coworkers in 1982 using the Singh index [3]. Generalized low bone mass and osteopenia in both the axial and peripheral skeleton in AIS have been reported in literature [5, 8, 10, 30]. Abnormal histomorphometric bone cell activity has been found in AIS bone biopsies [9]. In addition, low bone mass in AIS patients was likely to persist through to adulthood [6]. There is a growing concern that adolescents with idiopathic scoliosis may have a lower peak bone mass, thereby increasing the risk of developing osteoporosis and related complications in later life [6, 8]. However, the exact mechanisms and causes of the bone loss in AIS are not identified yet.
Receptor activator of nuclear factor-κB ligand (RANKL) is a potent stimulator of bone resorption by binding receptor activator of nuclear factor-κB (RANK) in the cell membrane of osteoclasts. In contrast, osteoprotegerin (OPG) is a soluble decoy receptor for RANKL which interferes with RANKL/RANK binding and inhibits the maturation and activation of osteoclasts and their precursors. The balance of RANKL/RANK and OPG has a central role in the regulation of bone remodeling events in diseases such as osteoporosis, glucocorticoid-induced osteoporosis, chronic inflammatory arthritis, hypogonadism, estrogen deficiency, bone marrow transplantation and the osteolytic bony metastasis of malignancies [2, 14. 17]. However, studies linking RANKL, OPG and bone mass of AIS are lacking. We aimed to study the relationship between serum concentration of soluble RANKL, serum level of OPG and bone mass in AIS girls and compared these to their levels in non-AIS controls.
Materials and methods
Subjects
Seventy-two newly diagnosed AIS girls aged between 11 and 14 years old were enrolled at the authors’ institution. Patients receiving any forms of prior treatment for scoliosis were excluded from the study. Sixty-four healthy girls of similar age range were recruited from local schools to serve as controls. All normal controls were also clinically examined to rule out any hidden scoliosis before entering the study. Subjects with a history of congenital deformities, neuromuscular disease, endocrine disease, skeletal dysplasia, connective tissue abnormalities or mental retardation were excluded from the study. All subjects and their parents gave informed consent before the examination and measurements. The study was approved by the Clinical Research Ethics Committee of the university and the hospital.
Evaluation of severity of scoliosis
A standard standing, whole spine, antero-posterior radiograph, was taken for each AIS patient at the first presentation. A standard technique was used for the measurement of the Cobb’s angle. If more than one curve was found, the more severe curve was selected for measurement. Curves of less than 10° were excluded.
Anthropometric measurement
Anthropometric measurements included body height and body weight. For AIS patients, corrected height was derived from Bjure’s formula (Log y = 0.011x − 0.177, where y is the loss of trunk height (cm) due to the deformed spine and x is the greatest Cobb angle of the primary curve) [6]. Body mass index (BMI) was determined by dividing weight (kg) by the square of the uncorrected height (m2).
Dual energy X-ray absorptiometry
Lumbar spinal bone mineral density (LSBMD) and femoral neck BMD (FNBMD), greater trochanter BMD (GTBMD) and Ward’s triangle BMD (WTBMD) of the non-dominant proximal femur were measured by dual-energy X-ray absorptiometry (DEXA) (XR-36; Norland Corporation, Fort Atkinson, WI, USA). LSBMD was measured in L1 through L4 with anterior-posterior view. The scoliotic curvature of the spine in AIS patients may present difficulties in measuring the spinal BMD reliably. To minimize this problem, the amount of rotation in patients with AIS was determined by pre-scanning of the spine and then the LSBMD was measured in the neutral position.
Measurement of serum RANKL and OPG
Serum was obtained from the routinely-drawn blood samples between 7 and 9 a.m., centrifuged immediately. The samples were kept at −80°C prior to determination of RANKL and OPG. RANKL levels were measured in serum by a sandwich ELISA (Immundiagnostik, Bensheim, Germany). As a first step, the blood sample and the biotinylated anti-RANKL detection antibody were pipetted into wells. Human RANKL, if present in the sample, binds to the precoated recombinant OPG and forms a sandwich with the detection antibody. After a washing step, which removed all nonspecific bound material, streptavidin-HRP conjugate was added to the wells. After removing unbound conjugate by washing, tetramethylbenzidine was added to the wells as substrate and the optical density (OD) at 450 nm was read on a standard ELISA plate reader, after an appropriate colour development period, to measure RANKL levels. The instrument’s software fit a calibrators-response curve to the OD results of the standards and calculated the RANKL of unknowns from the standard curve equation. We used five standards (0, 1.25, 2.5, 5, 10 pmol/l, respectively). The detection limit was 0.07 pmol/l and the dynamic range was 0.07–10 pmol/l. The intra-assay CV for RANKL measurement was 3–5% and interassay CV was 6–9%.
Serum OPG levels were measured by sandwich ELISA (Immundiagnostik, Bensheim, Germany) method. The assay includes two highly specific antibodies against OPG. The antibody is capable of neutralizing the biological activity of recombinant human OPG. The detection antibody was a biotin-labelled polyclonal antihuman OPG antibody derived from a goat, immunized with human recombinant OPG. The concentrations of serum OPG were calculated on the basis of the protein concentration as described by the manufacturer. The instrument’s software fit a calibrators-response curve to the OD results of the standards and calculated the OPG of unknowns from the standard curve equation. We used five standards (0, 1.1, 3.3, 10, 30 pmol/l, respectively). The detection limit was 0.14 pmol/l and the dynamic range was 0.14–30 pmol/l. The intra-assay coefficient of variation (CV) for OPG measurement was 8–10% and interassay CV was below 10%.
Statistical analysis
Statistical analysis was performed with SPSS 11.5 software for Windows (SPSS, Chicago, IL, USA). Data were expressed by mean ± standard deviation. Groups were compared by using the t test. Also, the correlations were presented using Pearson’s and Spearman’s correlation in each group, as appropriate. A P < 0.05 was regarded as statistically significant.
Results
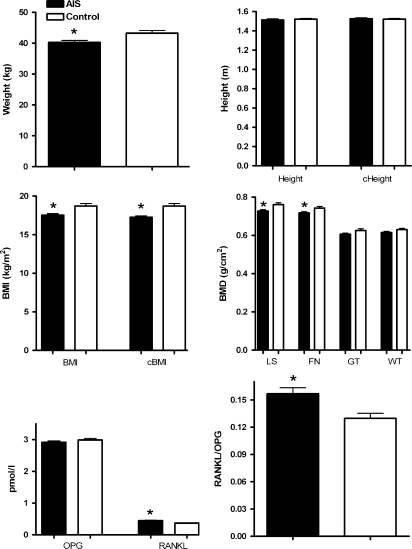
Figure 1 shows the results of the patients with AIS and the control individuals. The mean weight, BMI and corrected BMI in the patients were 40.3 ± 5.4 kg, 17.5 ± 1.6 kg/m2 and 17.3 ± 1.6 kg/m2, respectively; whereas, the mean was 43.3 ± 6.6 kg, 18.7 ± 2.6 kg/m2 and 18.7 ± 2.6 kg/m2 in the control group. The mean weight, BMI and corrected BMI in patients with AIS were decreased compared with that in the control individuals, respectively (P = 0.0039, P = 0.0016 and P = 0.0001). The mean LSBMD and FNBMD in the patients were 0.727 ± 0.06 and 0.718 ± 0.053 g/cm2, respectively; whereas, the mean was 0.761 ± 0.071 and 0.743 ± 0.069 g/cm2 in the control group, respectively. The mean LSBMD and FNBMD in patients with AIS were decreased compared with that in control individuals, respectively (P = 0.0029 and P = 0.0192, respectively). There was no significant difference in GTBMD and WTBMD between the AIS and the control subjects. The mean RANKL and RANKL to OPG ratio in the patients were 0.444 ± 0.130 pmol/l and 0.157 ± 0.058, respectively; whereas, the mean was 0.372 ± 0.093 pmol/l and 0.129 ± 0.045 in the control group, respectively. The mean RANKL and RANKL to OPG ratio in patients with AIS were increased compared with that in control subjects, respectively (P = 0.0004 and P = 0.0032, respectively). There was no significant difference in OPG between the AIS and the control subjects (P = 0.2849).
Fig. 1.
Results of the AIS patients and control subjects. (sec statistically significant difference, cHeight corrected height, cBMI corrected BMI)
The correlations of the parameters are shown in Tables 1 and 2. The chronological age was positively correlated to LSBMD and FNBMD and negatively correlated to RANKL and RANKL to OPG ratios in both groups. However, the BMI was not correlated to any parameters in both groups. The RANKL and RANKL to OPG ratios were negatively correlated to the LSBMD and serum OPG levels in both groups. Serum OPG levels were positively correlated to the LSBMD and FNBMD in both groups.
Table 1.
Correlations of the parameters in patients with AIS
| Age | BMI | cBMI | LSBMD | FNBMD | GTBMD | WTBMD | RANKL | OPG | RANKL/OPG | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | r | −0.072 | −0.075 | 0.665 | 0.642 | 0.254 | 0.170 | −0.719 | 0.327 | −0.703 | |
| P | 0.5500 | 0.5309 | 0.0000 | 0.0000 | 0.0314 | 0.1538 | 0.0000 | 0.0051 | 0.0000 | ||
| BMI | r | −0.072 | 0.997 | −0.036 | 0.021 | 0.188 | −0.007 | 0.074 | −0.000 | 0.181 | |
| P | 0.5500 | 0.0000 | 0.7667 | 0.8580 | 0.1130 | 0.9546 | 0.5343 | 0.9991 | 0.1281 | ||
| cBMI | r | −0.075 | 0.997 | −0.033 | 0.025 | 0.186 | 0.010 | 0.076 | −0.010 | 0.177 | |
| P | 0.5309 | 0.0000 | 0.7837 | 0.8321 | 0.1174 | 0.9351 | 0.5256 | 0.9331 | 0.1357 | ||
| LSBMD | r | 0.665 | −0.036 | −0.033 | 0.663 | 0.326 | 0.208 | −0.604 | 0.290 | −0.622 | |
| P | 0.0000 | 0.7667 | 0.7837 | 0.0000 | 0.0052 | 0.0800 | 0.0000 | 0.0134 | 0.0000 | ||
| FNBMD | r | 0.642 | 0.021 | 0.025 | 0.663 | 0.162 | 0.169 | −0.602 | 0.285 | −0.604 | |
| P | 0.0000 | 0.8580 | 0.8321 | 0.0000 | 0.1744 | 0.1551 | 0.0000 | 0.0152 | 0.0000 | ||
| GTBMD | r | 0.254 | 0.188 | 0.186 | 0.326 | 0.162 | 0.435 | −0.235 | 0.048 | −0.202 | |
| P | 0.0314 | 0.1130 | 0.1174 | 0.0052 | 0.1744 | 0.0001 | 0.0471 | 0.6895 | 0.0893 | ||
| WTBMD | r | 0.170 | −0.007 | 0.010 | 0.208 | 0.169 | 0.435 | −0.278 | 0.073 | −0.232 | |
| P | 0.1538 | 0.9546 | 0.9351 | 0.0800 | 0.1551 | 0.0001 | 0.0179 | 0.5416 | 0.0505 | ||
| RANKL | r | −0.719 | 0.074 | 0.076 | −0.604 | −0.602 | −0.235 | −0.278 | −0.400 | 0.950 | |
| P | 0.0000 | 0.5343 | 0.5256 | 0.0000 | 0.0000 | 0.0471 | 0.0179 | 0.0005 | 0.0000 | ||
| OPG | r | 0.327 | −0.000 | −0.010 | 0.290 | 0.285 | 0.048 | 0.073 | −0.400 | −0.617 | |
| P | 0.0051 | 0.9991 | 0.9331 | 0.0134 | 0.0152 | 0.6895 | 0.5416 | 0.0005 | 0.0000 | ||
| RANKL/OPG | R | −0.703 | 0.181 | 0.177 | −0.622 | −0.604 | −0.202 | −0.232 | 0.950 | −0.617 | |
| P | 0.0000 | 0.1281 | 0.1357 | 0.0000 | 0.0000 | 0.0893 | 0.0505 | 0.0000 | 0.0000 | ||
cBMI corrected BMI
Table 2.
Correlations of the parameters in control subjects
| Age | BMI | LSBMD | FNBMD | GTBMD | WTBMD | RANKL | OPG | RANKL/OPG | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | r | 0.191 | 0.659 | 0.376 | 0.228 | 0.301 | −0.677 | 0.575 | −0.734 | |
| P | 0.1315 | 0.0000 | 0.0022 | 0.0698 | 0.0156 | 0.0000 | 0.0000 | 0.0000 | ||
| BMI | r | 0.191 | 0.207 | 0.171 | −0.036 | 0.096 | −0.025 | −0.076 | −0.098 | |
| P | 0.1315 | 0.1002 | 0.1767 | 0.7777 | 0.4508 | 0.8452 | 0.5488 | 0.4357 | ||
| LSBMD | r | 0.659 | 0.207 | 0.695 | 0.350 | 0.531 | −0.397 | 0.424 | −0.510 | |
| P | 0.0000 | 0.1002 | 0.0000 | 0.0046 | 0.0000 | 0.0012 | 0.0005 | 0.0001 | ||
| FNBMD | r | 0.376 | 0.171 | 0.695 | 0.296 | 0.544 | −0.154 | 0.260 | −0.287 | |
| P | 0.0022 | 0.1767 | 0.0000 | 0.0176 | 0.0000 | 0.2246 | 0.0380 | 0.0225 | ||
| GTBMD | r | 0.228 | −0.036 | 0.350 | 0.296 | 0.496 | −0.041 | 0.068 | −0.114 | |
| P | 0.0698 | 0.7777 | 0.0046 | 0.0176 | 0.0000 | 0.7467 | 0.5926 | 0.3666 | ||
| WTBMD | r | 0.301 | 0.096 | 0.531 | 0.544 | 0.496 | −0.091 | 0.251 | −0.271 | |
| P | 0.0156 | 0.4508 | 0.0000 | 0.0000 | 0.0000 | 0.4733 | 0.0453 | 0.0312 | ||
| RANKL | r | −0.677 | −0.025 | −0.397 | −0.154 | −0.041 | −0.091 | −0.580 | 0.949 | |
| P | 0.0000 | 0.8452 | 0.0012 | 0.2246 | 0.7467 | 0.4733 | 0.0000 | 0.0000 | ||
| OPG | r | 0.575 | −0.076 | 0.424 | 0.260 | 0.068 | 0.251 | −0.580 | −0.829 | |
| P | 0.0000 | 0.5488 | 0.0005 | 0.0380 | 0.5926 | 0.0453 | 0.0000 | 0.0000 | ||
| RANKL/OPG | r | −0.734 | −0.098 | −0.510 | −0.287 | −0.114 | −0.271 | 0.949 | −0.829 | |
| P | 0.0000 | 0.4357 | 0.0001 | 0.0225 | 0.3666 | 0.0312 | 0.0000 | 0.0000 | ||
Discussion
A generalized low bone mass and osteopenia in both the axial and peripheral skeleton in AIS have been reported in literature [5, 8, 10, 30]. However, the exact mechanisms of bone loss in AIS patients are not fully identified. Reports have shown that the BMD in children can be affected by factors such as body weight, body height, physical activity, or nutrition status [18, 19, 24, 26]. Regular exercise has been reported to significantly increase peak bone mass [18, 24]. The relationship between a diagnosis of AIS and low body-weight may indicate disordered eating and is thus a cause for concern, particularly in the light of the well-established relationship between eating psychopathology and osteoporosis [27]. However, no significant difference was found in physical activity between the AIS and the controls [8]. Although the current study revealed the statistically significant differences of BMI between AIS and controls, the BMI was not correlated to any parameter in both groups. It is generally accepted that the BMD is influenced by the body weight, height, and BMI. The current study did not show any correlations of BMD with other factors such as chronological age, weight, body height, and BMI. However, the chronological age had significant correlations with BMD and RANKL/OPG system in both of the AIS and controls. We did not divide the AIS and control groups according to the chronological age because of narrow age range of the study groups. Therefore, these results should be confirmed further by a larger population based study.
RANKL is over-expressed and OPG is under-expressed in the tissues of active synovitis in inflammatory arthritis, and there is a general agreement about the fact that the imbalance of these two molecules has an important role in the bone loss and destruction in inflammatory arthritis [11, 15]. There have been many studies performed in regard to the relationship between circulating RANKL-OPG system and BMD. Gene therapy with human recombinant OPG has been shown to reverse established osteopenia in ovariectomized mice or in growing rats, supporting the role of OPG as the protective action in bone loss [4, 21]. Although conflicting, however, serum OPG levels show an inverse relationship with BMD in humans [25]. However, studies linking RANKL, OPG and bone mass of AIS are lacking. Therefore, we aimed to study the relationship between serum concentration of soluble RANKL, serum level of OPG and bone mass in AIS girls and compared it with those of healthy non-AIS controls.
In this study, we found out that serum OPG levels in AIS was not so much lower, but RANKL levels and RANKL to OPG ratio are much higher in AIS than in controls. The RANKL and RANKL to OPG ratios were negatively correlated to the LSBMD and serum OPG levels in both groups. Moreover, the RANKL, OPG and RANKL to OPG ratios were not correlated to the BMI. These results might impose the assumptions that the loss of BMD in AIS may be caused by the changes of the RANKL/OPG system rather than the effects of the weight and BMI loss. These results are described first in the present study. These findings mean that the imbalance and the disturbed interaction of RANKL and OPG may be an important cause and pathogenesis in reduced BMD in AIS.
Some potential limitations of this study should be considered. Firstly, the number of patients included was relatively small. To reflect the true levels of serum RANKL and OPG in bone, studies with larger patient numbers should be performed. Secondly, it is well known that the serum levels of OPG and RANKL are changed in some disease conditions. However, these levels are not specific for delineation of disease conditions. In the current study, the AIS patients revealed the reduction of RANKL levels. Nevertheless, with these changes of the RANKL and OPG serum levels, it might be difficult to distinguish the AIS from other disease conditions. Thirdly, we could not assess the correlation of inflammatory cytokines or bone turnover markers contributory to osteoclastogenesis and RANKL expression because we did not measure the serum levels of inflammatory cytokines and bone turnover markers such as interleukin-1, interleukin-6, tumor necrosis factor-α, osteocalcin and alkaline phosphatase. Further studies with measurement of inflammatory cytokines and bone turnover markers might support the relationship of these cytokines with serum OPG or RANKL levels in patients with AIS.
In summary, we attempted to determine the low BMD, to measure the serum levels of RANKL and OPG, and to find the correlation between BMD and serum RANKL, OPG levels and RANKL to OPG ratios in AIS. The serum RANKL levels and RANKL to OPG ratios were up-regulated in patients with AIS and tended to be related to LSBMD and FNBMD. Our data suggest that the imbalance of RANKL and OPG might be one of the mechanisms leading to low bone mass in AIS. When managing patients with AIS, clinicians should pay attention as low bone mass is common in AIS despite young age. Future therapies should be aimed for restoration of the unbalanced RANKL/OPG system in AIS patients.
Acknowledgments
K. T. Suh, S.-S. Lee and S. H. Hwang contributed equally to this study. This study was supported by Medical Research Institute Grant (2007-12), Pusan National University.
References
- 1.Ahn UM, Ahn NU, Nallamshetty L, Buchowski JM, Rose PS, Miller NH, Kostuik JP, Sponseller PD. The etiology of adolescent idiopathic scoliosis. Am J Orthop. 2002;31:387–395. [PubMed] [Google Scholar]
- 2.Baek KH, Oh KW, Lee WY, Tae HJ, Rhee EJ, Han JH, Cha BY, Kim YJ, Lee KW, Son HY, Kang SK, Kim CC, Kang MI. Changes in the serum sex steroids, IL-7 and RANKL-OPG system after bone marrow transplantation: influences on bone and mineral metabolism. Bone. 2006;39:1352–1360. doi: 10.1016/j.bone.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Burner WL, Badger VM, Sherman FC. Osteoporosis and acquired back deformities. J Pediatr Orthop. 1982;2:383–385. doi: 10.1097/01241398-198210000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Capparelli C, Morony S, Warmington K, Adamu S, Lacey D, Dunstan CR, Stouch B, Martin S, Kostenuik PJ. Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): a pharmacodynamic and pharmacokinetic analysis in rats. J Bone Miner Res. 2003;18:852–858. doi: 10.1359/jbmr.2003.18.5.852. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JC, Guo X. Osteopenia in adolescent idiopathic scoliosis. A primary problem or secondary to the spinal deformity? Spine. 1997;22:1716–1721. doi: 10.1097/00007632-199708010-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cheng JC, Guo X, Sher AH. Persistent osteopenia in adolescent idiopathic scoliosis. A longitudinal follow up study. Spine. 1999;24:1218–1222. doi: 10.1097/00007632-199906150-00008. [DOI] [PubMed] [Google Scholar]
- 7.Cheng JC, Leung SS, Lee WT, Lau JT, Maffulli N, Cheung AY, Chan KM. Determinants of axial and peripheral bone mass in Chinese adolescents. Arch Dis Child. 1998;78:524–530. doi: 10.1136/adc.78.6.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng JC, Qin L, Cheung CS, Sher AH, Lee KM, Ng SW, Guo X. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15:1587–1595. doi: 10.1359/jbmr.2000.15.8.1587. [DOI] [PubMed] [Google Scholar]
- 9.Cheng JC, Tang SP, Guo X, Chan CW, Qin L. Osteopenia in adolescent idiopathic scoliosis: a histomorphometric study. Spine. 2001;26:E19–E23. doi: 10.1097/00007632-200104150-00023. [DOI] [PubMed] [Google Scholar]
- 10.Cook SD, Harding AF, Morgan EL, Nicholson RJ, Thomas KA, Whitecloud TS, Ratner ES. Trabecular bone mineral density in idiopathic scoliosis. J Pediatr Orthop. 1987;7:168–174. doi: 10.1097/01241398-198703000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Crotti TN, Smith MD, Weedon H, Ahern MJ, Findlay DM, Kraan M, Tak PP, Haynes DR. Receptor activator NF-kappaB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis. 2002;61:1047–1054. doi: 10.1136/ard.61.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford DM, Bagnall KM, Clements CA, McFadden KD. Muscle spindles in the paraspinal musculature of patients with adolescent idiopathic scoliosis. Spine. 1988;13:461–465. doi: 10.1097/00007632-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Ford DM, Bagnall KM, McFadden KD, Greenhill BJ, Raso VJ. Paraspinal muscle imbalance in adolescent idiopathic scoliosis. Spine. 1984;9:373–376. doi: 10.1097/00007632-198405000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Garcia Palacios V, Robinson LJ, Borysenko CW, Lehmann T, Kalla SE, Blair HC. Negative regulation of RANKL-induced osteoclastic differentiation in RAW264.7 Cells by estrogen and phytoestrogens. J Biol Chem. 2005;280:13720–13727. doi: 10.1074/jbc.M410995200. [DOI] [PubMed] [Google Scholar]
- 15.Haynes DR, Barg E, Crotti TN, Holding C, Weedon H, Atkins GJ, Zannetino A, Ahern MJ, Coleman M, Roberts-Thomson PJ, Kraan M, Tak PP, Smith MD. Osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathies and osteoarthritis and normal controls. Rheumatology. 2003;42:123–134. doi: 10.1093/rheumatology/keg047. [DOI] [PubMed] [Google Scholar]
- 16.Herman R, Mixon J, Fisher A, Maulucci R, Stuyck J. Idiopathic scoliosis and the central nervous system: a motor control problem [The Harrington lecture 1983]. Scoliosis Research Society. Spine. 1985;10:1–14. doi: 10.1097/00007632-198501000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Hofbauer LC, Gori F, Riggs BL, Lacey DL, Dunstan CR, Spelsberg TC, Khosla S. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology. 1999;140:4382–4389. doi: 10.1210/en.140.10.4382. [DOI] [PubMed] [Google Scholar]
- 18.Ho S, Wong E, Chan SG, Lau J, Chan C, Leung PC. Determinants of peak bone mass in Chinese women aged 21–40 years. III. Physical activity and bone mineral density. J Bone Miner Res. 1997;12:1262–1271. doi: 10.1359/jbmr.1997.12.8.1262. [DOI] [PubMed] [Google Scholar]
- 19.Johnston CC, Jr, Miller JZ, Slemenda CW, Reister TK, Hui S, Christian JC, Peacock M. Calcium supplementation and increase in bone mineral density in children. New Engl J Med. 1992;327:82–87. doi: 10.1056/NEJM199207093270204. [DOI] [PubMed] [Google Scholar]
- 20.Kindsfater K, Lowe T, Lawellin D, Weinstein D, Akmakjian J. Levels of platelet calmodulin for the prediction of progression and severity of adolescent idiopathic scoliosis. J Bone Joint Surg. 1994;76-A:1186–1192. doi: 10.2106/00004623-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Kostenuik PJ, Bolon B, Morony S, Daris M, Geng Z, Carter C, Sheng J. Gene therapy with human recombinant osteoprotegerin reverses established osteopenia in ovariectomized mice. Bone. 2004;34:656–664. doi: 10.1016/j.bone.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Machida M, Dubousset J, Imamura Y, Iwaya T, Yamada T, Kimura J, Toriyama S. Pathogenesis of idiopathic scoliosis: SEPs in chicken with experimentally induced scoliosis and in patients with idiopathic scoliosis. J Pediatr Orthop. 1994;14:329–335. doi: 10.1097/01241398-199405000-00010. [DOI] [PubMed] [Google Scholar]
- 23.McCarrey JR, Abbott UK, Benson DR, Riggins RS. Genetics of scoliosis in chickens. J Hered. 1981;72:6–10. doi: 10.1093/oxfordjournals.jhered.a109428. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson BE, Westlin NE. Bone density in athletes. Clin Orthop. 1971;77:179–182. [PubMed] [Google Scholar]
- 25.Rogers A, Saleh G, Hannon RA, Greenfield D, Eastell R. Circulating estradiol and osteoprotegerin as determinants of bone turnover and bone density in postmenopausal women. J Clin Endocrinol Metab. 2002;87:4470–4475. doi: 10.1210/jc.2002-020396. [DOI] [PubMed] [Google Scholar]
- 26.Slemenda CW, Peacock M, Hui S, Zhou L, Johnston CC. Reduced rates of skeletal remodeling are associated with increased bone mineral density the development of peak skeletal mass. J Bone Miner Res. 1997;12:676–682. doi: 10.1359/jbmr.1997.12.4.676. [DOI] [PubMed] [Google Scholar]
- 27.Smith FM, Latchford G, Hall RM, Millner PA, Dickson RA. Indications of disordered eating behaviour in adolescent patients with idiopathic scoliosis. J Bone Joint Surg. 2002;84-B:392–394. doi: 10.1302/0301-620X.84B3.12619. [DOI] [PubMed] [Google Scholar]
- 28.Stilwell DL., Jr Structural deformities of vertebrae: Bone adaptation and modeling in experimental scoliosis and kyphosis. J Bone Joint Surg. 1962;44-A:611–634. [PubMed] [Google Scholar]
- 29.Suh KT, Lee SS, Kim SJ, Kim YK, Lee JS. Pineal gland metabolism in patients with adolescent idiopathic scoliosis. J Bone Joint Surg. 2007;89-B:66–71. doi: 10.1302/0301-620X.89B1.18058. [DOI] [PubMed] [Google Scholar]
- 30.Thomas KA, Cook SD, Skalley TC, Renshaw SV, Makuch RS, Gross M, Whitecloud TS, 3rd, Bennett JT. Lumbar spine and femoral neck bone mineral density in idiopathic scoliosis: a follow up study. J Pediatr Orthop. 1992;12:235–240. doi: 10.1097/01241398-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Wang ED, Drummond DS, Dormans JP, Moshang T, Davidson RS, Gruccio D. Scoliosis in patients treated with growth hormone. J Pediatr Orthop. 1997;17:708–711. doi: 10.1097/00004694-199711000-00003. [DOI] [PubMed] [Google Scholar]