Abstract
A sulfur-regulated gene (cysA) that encodes the membrane-associated ATP-binding protein of the sulfate transport system of the cyanobacterium Synechococcus sp. strain PCC 7942 was recently isolated and sequenced. Adjacent to cysA and transcribed in the opposite direction is a gene encoding the sulfate-binding protein (sbpA). Two other genes, cysT and cysW, encode proteins that may form a channel for the transport of sulfate across the cytoplasmic membrane. A fourth gene, cysR, located between cysT, and cysW, encodes a polypeptide that has some homology to a family of prokaryotic regulatory proteins. Mutant strains in which cysA, cysT, or cysW was interrupted by a drug resistance marker were not viable when grown with sulfate as the sole sulfur source and exhibited essentially no sulfate uptake. In contrast, sbpA and cysR mutants grew on sulfate, although they did not exhibit the 20-fold increase in the Vmax (concentration of sulfate at half-maximal transport rate) for sulfate transport characteristic of wild-type cells grown under sulfur-limiting conditions. Three of the sulfur-regulated genes in Synechococcus sp. strain PCC 7942 are similar to genes encoded by the chloroplast genome of the primitive plant Marchantia polymorpha. These data suggest that a sulfate transport system similar to that of Synechococcus sp. strain PCC 7942 may exist in the chloroplast envelope of photosynthetic eukaryotes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Oxender D. L. Bacterial periplasmic binding protein tertiary structures. J Biol Chem. 1989 Sep 25;264(27):15739–15742. [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Ames G. F., Nikaido K., Groarke J., Petithory J. Reconstitution of periplasmic transport in inside-out membrane vesicles. Energization by ATP. J Biol Chem. 1989 Mar 5;264(7):3998–4002. [PubMed] [Google Scholar]
- Batut J., Daveran-Mingot M. L., David M., Jacobs J., Garnerone A. M., Kahn D. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 1989 Apr;8(4):1279–1286. doi: 10.1002/j.1460-2075.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Brennan R. G., Matthews B. W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989 Feb 5;264(4):1903–1906. [PubMed] [Google Scholar]
- Churchill M. E., Suzuki M. 'SPKK' motifs prefer to bind to DNA at A/T-rich sites. EMBO J. 1989 Dec 20;8(13):4189–4195. doi: 10.1002/j.1460-2075.1989.tb08604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- Dassa E., Hofnung M. Sequence of gene malG in E. coli K12: homologies between integral membrane components from binding protein-dependent transport systems. EMBO J. 1985 Sep;4(9):2287–2293. doi: 10.1002/j.1460-2075.1985.tb03928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebright R. H., Cossart P., Gicquel-Sanzey B., Beckwith J. Mutations that alter the DNA sequence specificity of the catabolite gene activator protein of E. coli. Nature. 1984 Sep 20;311(5983):232–235. doi: 10.1038/311232a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Green L. S., Grossman A. R. Changes in sulfate transport characteristics and protein composition of Anacystis nidulans R2 during sulfur deprivation. J Bacteriol. 1988 Feb;170(2):583–587. doi: 10.1128/jb.170.2.583-587.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. S., Laudenbach D. E., Grossman A. R. A region of a cyanobacterial genome required for sulfate transport. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1949–1953. doi: 10.1073/pnas.86.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths L., Cole J. A. Lack of redox control of the anaerobically-induced nirB+ gene of Escherichia coli K-12. Arch Microbiol. 1987 May;147(4):364–369. doi: 10.1007/BF00406134. [DOI] [PubMed] [Google Scholar]
- Hellinga H. W., Evans P. R. Nucleotide sequence and high-level expression of the major Escherichia coli phosphofructokinase. Eur J Biochem. 1985 Jun 3;149(2):363–373. doi: 10.1111/j.1432-1033.1985.tb08934.x. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Haag P. D., Nikaido K., Ardeshir F., Garcia G., Ames G. F. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982 Aug 19;298(5876):723–727. doi: 10.1038/298723a0. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hryniewicz M., Sirko A., Pałucha A., Böck A., Hulanicka D. Sulfate and thiosulfate transport in Escherichia coli K-12: identification of a gene encoding a novel protein involved in thiosulfate binding. J Bacteriol. 1990 Jun;172(6):3358–3366. doi: 10.1128/jb.172.6.3358-3366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulanicka M. D., Garrett C., Jagura-Burdzy G., Kredich N. M. Cloning and characterization of the cysAMK region of Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):322–327. doi: 10.1128/jb.168.1.322-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isihara H., Hogg R. W. Amino acid sequence of the sulfate-binding protein from Salmonella typhimurium LT2. J Biol Chem. 1980 May 25;255(10):4614–4618. [PubMed] [Google Scholar]
- Jeanjean R., Broda E. Dependence of sulphate uptake by Anacystis nidulans on energy, on osmotic shock and on sulphate stravation. Arch Microbiol. 1977 Jul 26;114(1):19–23. doi: 10.1007/BF00429625. [DOI] [PubMed] [Google Scholar]
- Johann S., Hinton S. M. Cloning and nucleotide sequence of the chlD locus. J Bacteriol. 1987 May;169(5):1911–1916. doi: 10.1128/jb.169.5.1911-1916.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohchi T., Shirai H., Fukuzawa H., Sano T., Komano T., Umesono K., Inokuchi H., Ozeki H., Ohyama K. Structure and organization of Marchantia polymorpha chloroplast genome. IV. Inverted repeat and small single copy regions. J Mol Biol. 1988 Sep 20;203(2):353–372. doi: 10.1016/0022-2836(88)90004-6. [DOI] [PubMed] [Google Scholar]
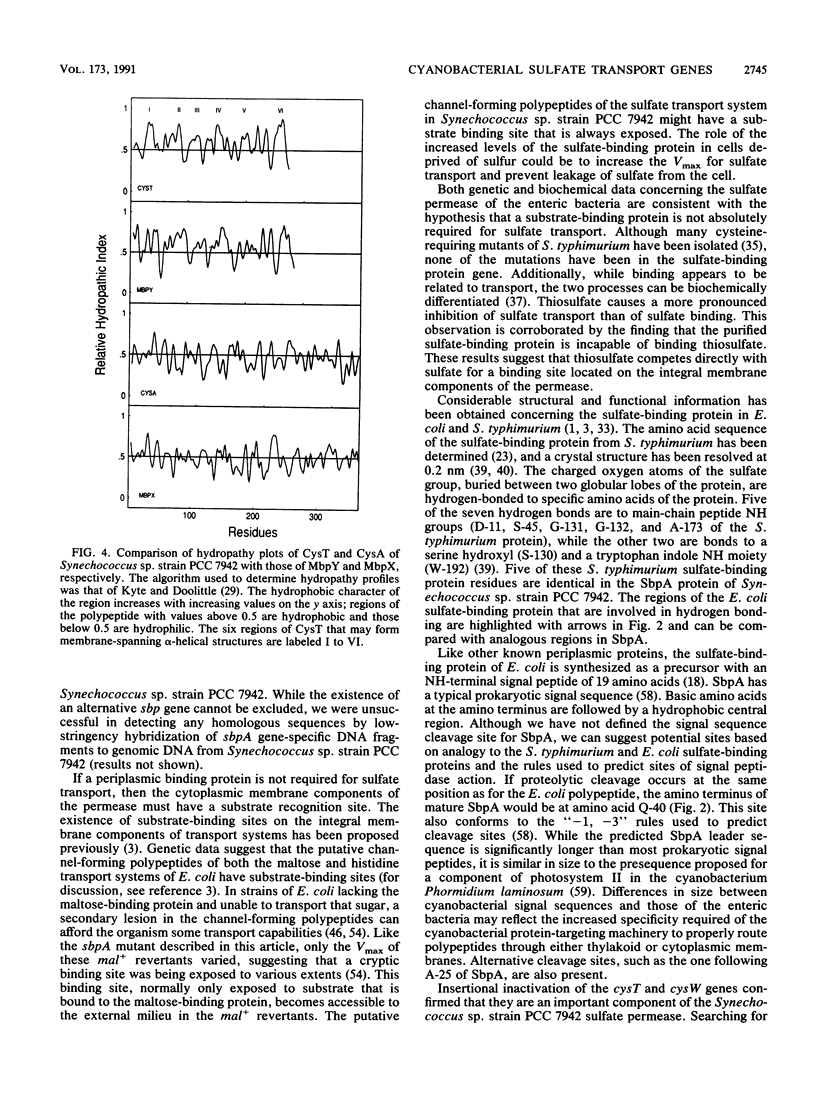
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laudenbach D. E., Reith M. E., Straus N. A. Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol. 1988 Jan;170(1):258–265. doi: 10.1128/jb.170.1.258-265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B., Pear M. R., McCammon J. A., Quiocho F. A. Hinge-bending in L-arabinose-binding protein. The "Venus's-flytrap" model. J Biol Chem. 1982 Feb 10;257(3):1131–1133. [PubMed] [Google Scholar]
- Mizobuchi K, Demerec M, Gillespie D H. Cysteine Mutants of Salmonella Typhimurium. Genetics. 1962 Nov;47(11):1617–1627. doi: 10.1093/genetics/47.11.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota N., Galsworthy P. R., Pardee A. B. Genetics of sulfate transport by Salmonella typhimurium. J Bacteriol. 1971 Mar;105(3):1053–1062. doi: 10.1128/jb.105.3.1053-1062.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B., Prestidge L. S., Whipple M. B., Dreyfuss J. A binding site for sulfate and its relation to sulfate transport into Salmonella typhimurium. J Biol Chem. 1966 Sep 10;241(17):3962–3969. [PubMed] [Google Scholar]
- Pardee A. B. Purification and properties of a sulfate-binding protein from Salmonella typhimurium. J Biol Chem. 1966 Dec 25;241(24):5886–5892. [PubMed] [Google Scholar]
- Pastan I., Adhya S. Cyclic adenosine 5'-monophosphate in Escherichia coli. Bacteriol Rev. 1976 Sep;40(3):527–551. doi: 10.1128/br.40.3.527-551.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugrath J. W., Quiocho F. A. Sulphate sequestered in the sulphate-binding protein of Salmonella typhimurium is bound solely by hydrogen bonds. Nature. 1985 Mar 21;314(6008):257–260. doi: 10.1038/314257a0. [DOI] [PubMed] [Google Scholar]
- Pflugrath J. W., Quiocho F. A. The 2 A resolution structure of the sulfate-binding protein involved in active transport in Salmonella typhimurium. J Mol Biol. 1988 Mar 5;200(1):163–180. doi: 10.1016/0022-2836(88)90341-5. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Prossnitz E., Nikaido K., Ulbrich S. J., Ames G. F. Formaldehyde and photoactivatable cross-linking of the periplasmic binding protein to a membrane component of the histidine transport system of Salmonella typhimurium. J Biol Chem. 1988 Dec 5;263(34):17917–17920. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scripture J. B., Voelker C., Miller S., O'Donnell R. T., Polgar L., Rade J., Horazdovsky B. F., Hogg R. W. High-affinity L-arabinose transport operon. Nucleotide sequence and analysis of gene products. J Mol Biol. 1987 Sep 5;197(1):37–46. doi: 10.1016/0022-2836(87)90607-3. [DOI] [PubMed] [Google Scholar]
- Sirko A., Hryniewicz M., Hulanicka D., Böck A. Sulfate and thiosulfate transport in Escherichia coli K-12: nucleotide sequence and expression of the cysTWAM gene cluster. J Bacteriol. 1990 Jun;172(6):3351–3357. doi: 10.1128/jb.172.6.3351-3357.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. J., Prossnitz E., Ames G. F. Role of the intercistronic region in post-transcriptional control of gene expression in the histidine transport operon of Salmonella typhimurium: involvement of REP sequences. Mol Microbiol. 1988 Jan;2(1):141–152. doi: 10.1111/j.1365-2958.1988.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Surin B. P., Rosenberg H., Cox G. B. Phosphate-specific transport system of Escherichia coli: nucleotide sequence and gene-polypeptide relationships. J Bacteriol. 1985 Jan;161(1):189–198. doi: 10.1128/jb.161.1.189-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treptow N. A., Shuman H. A. Genetic evidence for substrate and periplasmic-binding-protein recognition by the MalF and MalG proteins, cytoplasmic membrane components of the Escherichia coli maltose transport system. J Bacteriol. 1985 Aug;163(2):654–660. doi: 10.1128/jb.163.2.654-660.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Inokuchi H., Shiki Y., Takeuchi M., Chang Z., Fukuzawa H., Kohchi T., Shirai H., Ohyama K., Ozeki H. Structure and organization of Marchantia polymorpha chloroplast genome. II. Gene organization of the large single copy region from rps'12 to atpB. J Mol Biol. 1988 Sep 20;203(2):299–331. doi: 10.1016/0022-2836(88)90002-2. [DOI] [PubMed] [Google Scholar]
- Unden G., Guest J. R. Isolation and characterization of the Fnr protein, the transcriptional regulator of anaerobic electron transport in Escherichia coli. Eur J Biochem. 1985 Jan 2;146(1):193–199. doi: 10.1111/j.1432-1033.1985.tb08638.x. [DOI] [PubMed] [Google Scholar]
- Wallace T. P., Stewart A. C., Pappin D., Howe C. J. Gene sequence for the 9 kDa component of Photosystem II from the cyanobacterium Phormidium laminosum indicates similarities between cyanobacterial and other leader sequences. Mol Gen Genet. 1989 Apr;216(2-3):334–339. doi: 10.1007/BF00334373. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Steitz T. A. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 A resolution. J Mol Biol. 1987 Nov 20;198(2):311–326. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]




