Abstract
Biomaterial-mediated gene delivery has recently emerged as a promising alternative to conventional gene transfer technologies that focus on direct delivery of viral vectors or DNA-polymer/matrix complexes. However, biomaterial-based strategies have primarily targeted transient gene expression vehicles, including plasmid DNA and adenovirus particles. This study expands on this work by characterizing biomaterial properties conducive to the surface immobilization of retroviral particles and subsequent transduction of mammalian cells at the cell-material interface. Self-assembled monolayers (SAMs) of functionally-terminated alkanethiols on gold were used to establish biomaterial surfaces of defined chemical composition. Gene transfer was observed to be greater than 90% on NH2-terminated surfaces, approximately 50% on COOH-functionalized surfaces, and undetectable on CH3-terminated SAMs, similar to controls of tissue culture-treated polystyrene. Gene delivery via the NH2-SAM was further characterized as a function of coating time, virus concentration, and cell seeding density. Finally, SAM-mediated gene delivery was comparable to fibronectin- and poly-L-lysine-based methods for gene transfer. This work is significant to establishing safe and effective gene therapy strategies, developing efficient methods for gene delivery, and supporting recent progress in the field of biomaterial-mediated gene transfer.
Keywords: fibroblast, genetic engineering, gene therapy, gene transfer, self-assembly, alkanethiol
INTRODUCTION
Genetic engineering of mammalian cells is central to numerous strategies for disease treatment, tissue regeneration, and the study of protein function and cellular processes [1-3]. Conventional methods for gene delivery include both in vitro and in vivo gene transfer based on viral vectors or DNA-polymer/matrix complexes. In vitro gene transfer typically involves the direct transfection or transduction of cultured cells that are subsequently analyzed for transgene activity and/or implanted for a therapeutic purpose. In vivo gene therapy involves the direct application of the gene carrier to the injured or diseased tissue. However, these approaches are often limited by inefficient transgene delivery and poor specificity. Recent attempts to overcome these limitations have focused on biomaterial-mediated gene delivery, wherein the gene carrier is immobilized to, or encapsulated within, a biomaterial support [1-3]. The hybrid gene-activated biomaterial is then seeded with cells in vitro or implanted. By co-localizing the gene delivery vehicle and cell adhesion, this method enhances gene transfer and specifically targets cells at the biomaterial interface, thereby reducing the risks associated with direct injection of the gene carrier. Therefore this approach provides several advantages over conventional gene delivery modalities, including reduced cytotoxicity and immunogenicity of freely diffusible gene carriers, limited ectopic transgene expression in neighboring tissues, improved stability of the gene carrier, and controlled levels of gene transfer and expression.
Strategies for the integration of biomaterials and gene delivery have targeted both viral and plasmid DNA-based gene carriers. Fang et al. originally developed this method in the form of a gene-activated matrix (GAM) in which plasmid DNA was loaded onto collagen sponges prior to implantation into segmental bone defects [4]. Plasmid DNA was later incorporated into poly(lactide-co-glycolide) (PLG) scaffolds with tunable degradation properties, allowing for sustained release of DNA from the scaffold for up to 1 month [5]. Subsequently, numerous studies have evaluated strategies for controlled release of plasmid DNA from polymeric scaffolds or immobilization of DNA at biomaterial surfaces [1]. Methods for plasmid release have primarily focused on DNA incorporation into biodegradable polymers, including collagen [4,6], hyaluronan [7,8], PLG [5,9], poly(ethylene glycol) [10], and ethyl vinyl-co-acetate [11]. Immobilization approaches typically utilize the interaction of DNA with cationic agents, including poly-L-lysine [12], polyethylenimine [13-15], chitosan [16], or dendrimers such as polyamidoamine [17]. More recently, efforts have focused on incorporating the high gene transfer efficiencies of viral vectors into biomaterial-based gene delivery. For example, adenovirus particles have been suspended in hydrogel microspheres of fibrin [18], collagen [18], alginate [19], and PLGA [20,21] and implanted for controlled delivery in vivo. Additionally, Schwarz and colleagues have developed a technique for freeze-drying recombinant adeno-associated virus particles onto implants to enhance tissue repair [22].
Although many strategies exist for biomaterial-mediated gene therapy with plasmid DNA or adenoviral particles, both of these methods generate only transient transgene expression. There are many examples of biomedical applications which require prolonged or sustained expression of the transgene. Therefore, an unfulfilled need exists for methods that immobilize or release gene carriers that permanently modify the cellular DNA, such as retroviruses and lentiviruses. In contrast to adeno- and adeno-associated viruses, these particles consist of an unstable lipid bilayer and RNA genome that present unique challenges for incorporation into biomaterials. Retrovirus immobilization to human plasma fibronectin or recombinant fibronectin fragments has been widely utilized to enhance gene delivery to several cell types [23-25]. However, fibronectin is an intrinsically bioreactive molecule that directs confounding effects on cell differentiation and proliferation [26-28]. Cationic and anionic polymers have been used to form large virus-polymer complexes and increase the rate of virus sedimentation onto cultured cells [29-31]. Building on these results, other studies have analyzed the effects of cationic surfaces or molecules to regulate the immobilization and delivery of negatively charged retrovirus particles [32-34]. The present study expands on this work by evaluating the effects of surface chemistry on biomaterial-mediated gene delivery.
Self-assembled monolayers (SAMs) are a robust, controlled, and stable biomaterial for analyzing the effects of surface chemistry on biological phenomena [35]. SAMs have been used extensively to study and control protein adsorption and cell adhesion and function in vitro and in vivo. More recently, plasmid DNA has been immobilized onto SAMs for biomaterial-directed gene delivery [36-38]. In the present work, we demonstrate SAM chemistry-dependent effects on retrovirus immobilization and gene delivery.
MATERIALS AND METHODS
Cell Culture
NIH3T3 murine fibroblasts (CRL-1658, American Type Culture Collection, Manassas, VA) were cultured DMEM, 10% fetal bovine serum, 100 U/ml penicillin G sodium, and 100 μg/ml streptomycin sulfate in a humidified 5% CO2 atmosphere at 37 °C. Cell culture media and antibiotics were obtained from Invitrogen (Carlsbad, CA), fetal bovine serum was purchased from Hyclone (Logan, UT), and all other cell culture supplements and reagents were acquired from Sigma (St. Louis, MO).
Self-Assembled Monolayers
Alkanethiols 1-dodecanethiol (HS-(CH2)11-CH3) and 11-mercaptoundecanoic acid (HS-(CH2)10-COOH) were purchased from Aldrich Chemical (Milwaukee, WI) and used as received. The amine-terminated alkanethiol 12-amino-1-mercaptododecane (HS-(CH2)12-NH2) was synthesized and characterized by our group [39]. SAMs of their respective alkanethiols are referred to hereafter as CH3, COOH, and NH2. Gold-coated substrates (35 mm tissue culture-treated polystyrene dishes) were prepared by sequential deposition of optically transparent films of 75 Å Ti and 150 Å Au using an electron beam evaporator (Thermionics, Hayward, CA) at a deposition rate of 2 Å/s and a chamber base-pressure of approximately 2×10-6 Torr.
For SAM assembly, freshly prepared Au-coated substrates were immersed overnight in alkanethiol solutions (1.0 mM in absolute ethanol). SAMs were validated by contact angle goniometry and X-ray photoelectron spectroscopy [39]. Following assembly, SAMs were rinsed with 95% ethanol and equilibrated in PBS for 10 min before addition of viral supernatants or matrix molecules. To study the effects of extracellular matrix molecules on retrovirus immobilization, SAMs were incubated with Pronectin (Sigma), bovine dermal type I collagen (Cohesion, Palo Alto, CA), human plasma fibronectin, or poly-L-lysine (MW: 70 kDa-150 kDa) at 20 μg/ml in PBS for 1 hr. Matrix-coated SAMs were washed twice in PBS prior to exposure to viral supernatant and cell seeding.
Retrovirus Production
Retroviral supernatant was produced with the pTJ66 vector from a stable ΦNX amphotropic producer cell line. The pTJ66 retroviral vector uses the promoter activity of the 5′ long terminal repeat (LTR) followed by an internal ribosomal entry site (IRES) to express a zeocin resistance-enhanced green fluorescent protein fusion protein (Zeo(r):eGFP), allowing for noninvasive analysis of transduction efficiency [40]. Plasmid DNA was purified from transformed E. coli using Megaprep kits from Qiagen (Valencia, CA). Helper-virus free ΦNX amphotropic producer cells were transiently transfected with plasmid DNA as previously described [41]. Four days following transfection, ΦNX cells were cultured in growth media (DMEM, 10% fetal bovine serum, 100 U/ml penicillin G sodium, 100 μg/ml streptomycin sulfate) supplemented with 200 μg/ml zeocin. After two weeks of antibiotic selection, individual zeocin-resistant colonies were isolated and characterized for production of pTJ66 retrovirus. Promising clones were expanded and frozen. For retrovirus collection, stable producer cells were thawed and expanded. Once the cells reached confluence, media was replaced with fresh growth media and dishes were transferred to a humidified 5% CO2 atmosphere at 32 °C for enhanced stability of retroviral particles [42]. Retroviral supernatants were collected at every 24 hr for up to 6 days, filtered through a 0.45 μm cellulose acetate filter, aliquoted, snap frozen, and stored at -80 °C until use. Viral titers were determined to be 1-2 × 106 colony-forming units per ml, as determined by the method of Galipeau et al [43].
Retroviral Transduction
Retroviral supernatant was thawed and added to biomaterial surfaces (2 ml per 35 mm dish). Samples were incubated at 32 °C for enhanced stability of retroviral particles for 16 hr, unless noted otherwise. Following incubation, surfaces were washed twice in PBS, and NIH3T3 fibroblasts were seeded at a density of 10,000 cells / cm2. At 72 hours post-seeding, cells were harvested via trypsinization and assessed for eGFP expression with a Becton Dickinson LSR benchtop flow cytometer.
Data Analysis
Data are reported as mean ± standard error of the mean (SEM), and are representative results of experiments performed at least three times. Statistical comparisons using SYSTAT 8.0 were based on an analysis of variance (ANOVA) and Tukey's test for pairwise comparisons, with a p-value < 0.05 considered significant.
RESULTS
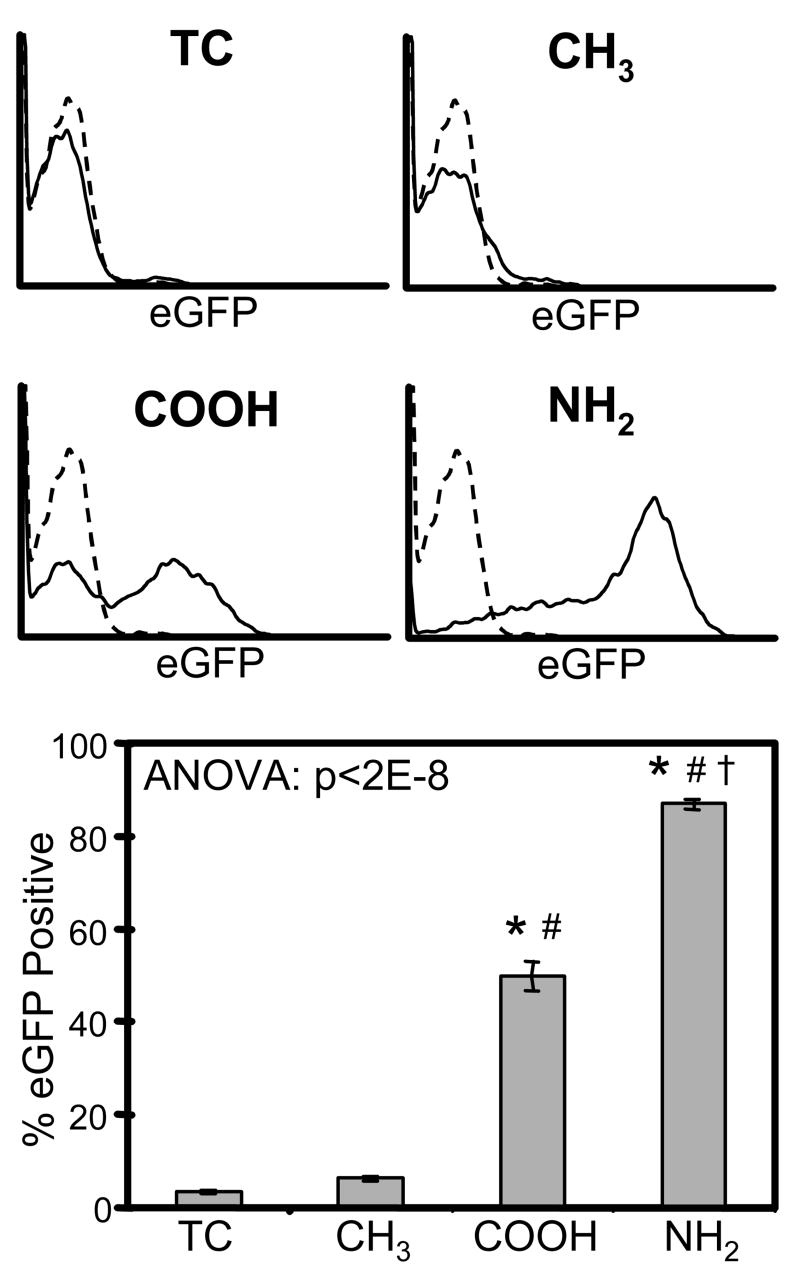
To test the effects of surface chemistry on retrovirus immobilization to biomaterial surfaces, eGFP-carrying retroviral supernatants were incubated on CH3, COOH, or NH2 SAMs or tissue culture-treated polystyrene control (TC) for 16 hr. Surfaces were then washed thoroughly with PBS and seeded with NIH3T3 murine fibroblasts. Expression of eGFP was measured by flow cytometry 72 hr later as an indicator of retrovirus immobilization and biomaterial-mediated gene transfer (Figure 1). In contrast to other studies [32-34], we did not detect significant levels of retrovirus gene transfer on TC. Similarly, cells seeded on the hydrophobic CH3 surface did not show detectable levels of eGFP expression. However, the COOH surface, which is negatively charged at physiologic pH of 7.2, demonstrated substantial levels of retroviral gene delivery, transducing ∼50% of cells. Notably, the positively charged NH2 surface produced the highest level of eGFP expression, corresponding to the efficient transduction of ∼90% of the cell population. These results demonstrate significant effects of biomaterial surface chemistry on retrovirus immobilization and transduction efficiency and show that gene delivery via the NH2 surface is an efficient means for genetic engineering of mammalian cells.
Figure 1.
Biomaterial surface chemistry modulates biomaterial-mediated retroviral gene delivery. Substrates where incubated with retroviral supernatant for 16 hr prior to seeding with NIH3T3 fibroblasts. Gene transduction was measured by flow cytometry for eGFP expression at 72 hr after cell seeding. TC = tissue culture-treated polystyrene. * vs. TC, # vs. CH3, † vs. COOH (p<0.05).
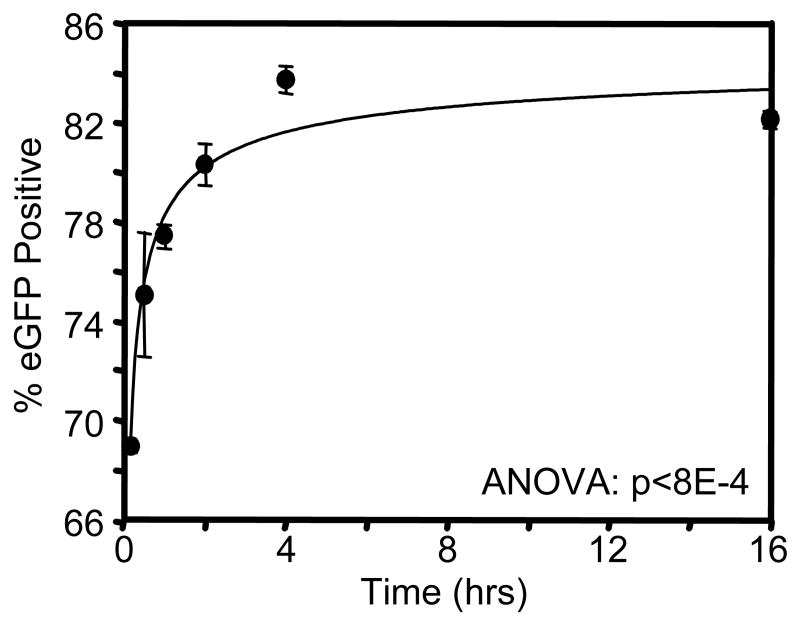
We further characterized gene delivery by the NH2 SAM by varying the incubation time with retroviral supernatants from 15 min to 16 hr (Figure 2). Freshly thawed virus was added to samples at staggered timepoints, such that samples were simultaneously washed with PBS and seeded with cells following the indicated incubation time. Interestingly, gene transfer efficiencies reached nearly 70% of cells following only 15 min of incubation with virus, indicating that neither diffusion of virus particles to the surface or the kinetics of the virus immobilization are substantially ratelimiting in this process. Transduction efficiencies gradually increased from ∼70% to ∼85% as incubation times increased from 15 min to 4 hr. Incubation times longer than 4 hr, up to 16 hr, did not significantly affect transduction efficiency. These results suggest that although the majority of our experiments were performed following 16-hr incubations, much shorter periods are sufficient to support retrovirus immobilization and efficient gene delivery.
Figure 2.
Duration of retrovirus incubation with substrate influences retrovirus immobilization and gene transfer activity on NH2 SAM. Retroviral supernatants were incubated on NH2 SAMs for different time periods prior to cell seeding. Gene transfer was measured by flow cytometry for eGFP expression at 72 hr after cell seeding.
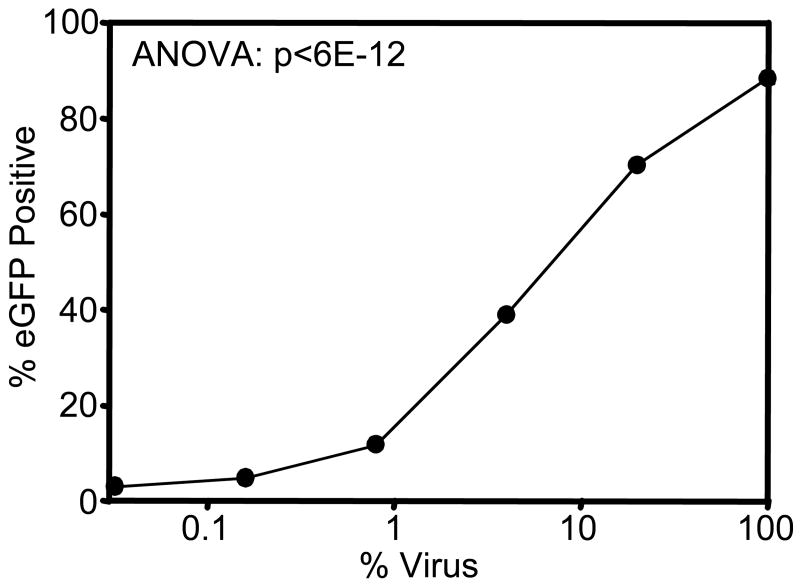
The effect of virus concentration on virus immobilization and activity on the NH2 SAM was studied by diluting viral stocks in fresh growth media prior to incubation with the NH2 surface (Figure 3). The titer of original virus stocks was determined to be 1-2 × 106 colony-forming units per ml, by a previously described method [43]. Transduction efficiency decreased with virus supernatant concentration for up to a 100-fold dilution, and dilution of the virus below 1% resulted in undetectable levels of gene transfer. The ability of the NH2 surface to promote detectable levels of gene transfer following 100-fold dilution of viral supernatants underscores the potency of this method in conferring efficient gene delivery.
Figure 3.
Biomaterial-assisted gene transfer is dependent on viral supernatant titer. Retroviral supernatants were diluted with growth media prior to incubation on the NH2 SAM for 16 hr prior to cell seeding. Gene transfer was measured by flow cytometry for eGFP expression at 72 hr post-seeding.
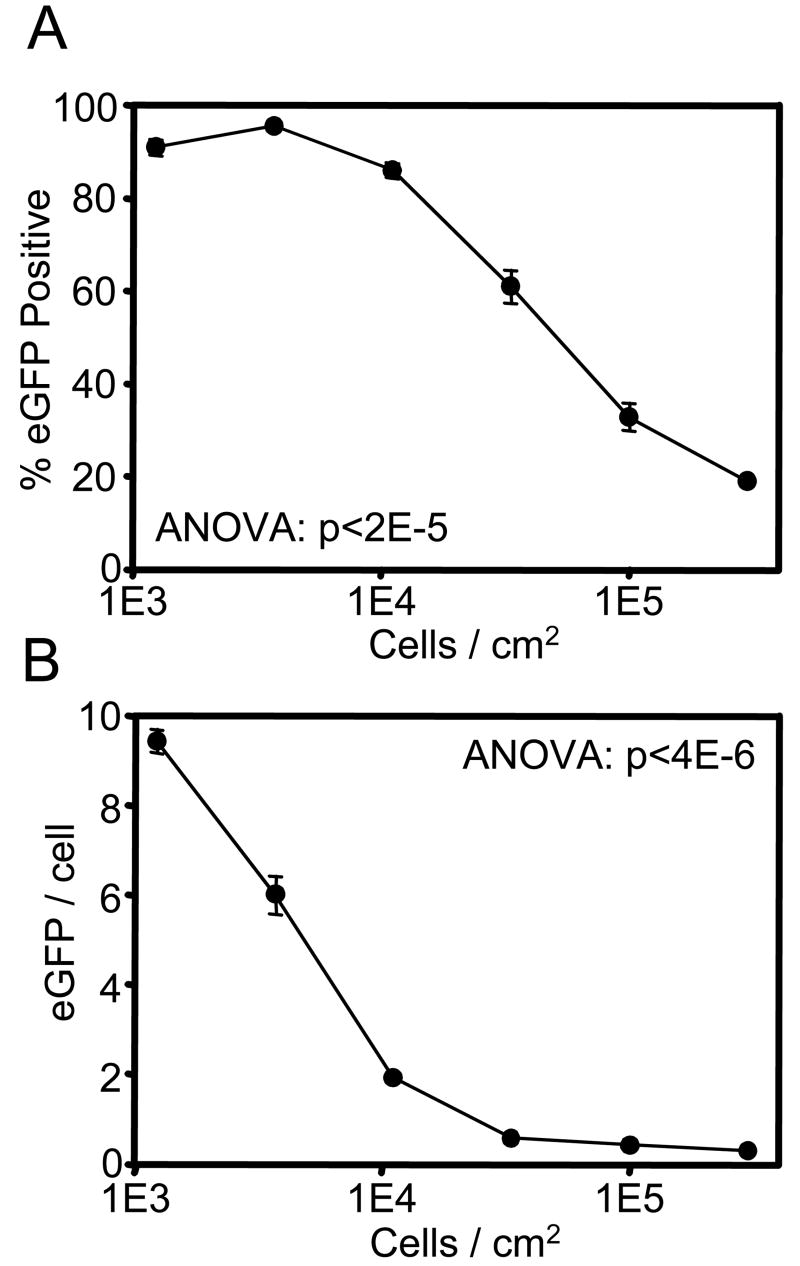
Transduction by retroviral particles is limited to actively dividing cells, as breakdown of the nuclear membrane is necessary for virus integration and transgene expression [44]. Therefore, we examined the role of cell density on transduction efficiency to determine the effect of contact-mediated growth inhibition on biomaterial-mediated gene delivery (Figure 4A). At low densities (<10,000 cells/cm2), cell-cell contact is minimal and cell density has no effect on transduction efficiency. However, the fraction of transduced cells dropped steadily as cell density increased. Interestingly, the mean level of eGFP fluorescence per eGFP-positive cell followed a similar trend as a function of cell density, although slightly shifted to lower cell densities (Figure 4B). This is indicative of increases in retroviral copy number per infected cell as cell density decreased. In fact, a similar correlation between transduction efficiency and mean eGFP fluorescence was observed when varying incubation time (Figure 2) and virus concentration (Figure 3). These results demonstrate control of gene delivery and level of gene expression through biomaterial-mediated gene transfer.
Figure 4.
Cell seeding density modulates biomaterial-mediated retroviral gene delivery. Retrovirus supernatants were incubated on NH2 SAMs for 16 hr prior to seeding with the indicated density of NIH3T3 fibroblasts. (A) The fraction of eGFP-positive cells and (B) the intensity of eGFP expression per eGFP-positive cell, as measured by flow cytometry at 72 hr post-seeding, are plotted as a function of cell seeding density.
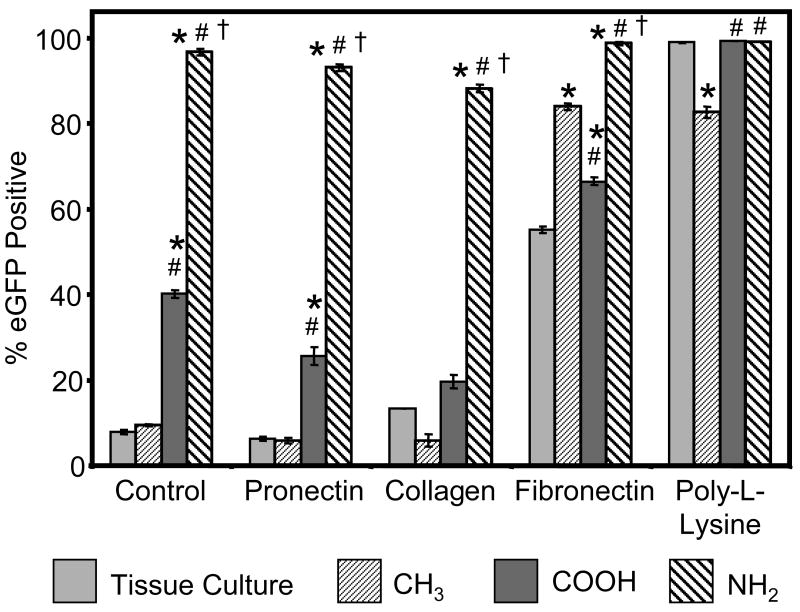
Finally, we analyzed the effects of pre-coating surfaces with extracellular matrix molecules on retrovirus immobilization and gene delivery (Figure 5). These experiments were performed to determine the ability of retroviral particles to immobilize onto surfaces that had been functionalized with bioactive molecules prior to retroviral exposure. Additionally, we compared transduction efficiencies on the NH2 SAM to other surface modifications known to immobilize retrovirus, including coating with fibronectin [23-25] or poly-L-lysine [32]. It is important to note that the retroviral supernatant is prepared in growth media containing 10% fetal bovine serum, so all of the experiments described above were also performed in the presence of surface-adsorbed serum proteins. Passive adsorption of type I bovine collagen or Pronectin, a synthetic RGD-containing molecule, had no effect on biomaterial-mediated gene transfer. This finding suggests that substantial modification of the biomaterial is possible without disrupting the surface-retrovirus interaction. Significant levels of gene transfer were detected on fibronectin-coated tissue culture polystyrene, as described previously [25]. However, fibronectin-assisted gene transfer exhibited surface chemistry-dependent behavior with highest transduction efficiencies on the NH2 SAM. Interestingly, transduction efficiency on the fibronectin-coated CH3 surface was higher than both the fibronectin-coated COOH SAM and TC surface, in contrast to the results on all other surface treatments. We attribute this result to surface chemistry-dependent conformational changes in the structure of adsorbed fibronectin that influence biological activity [27,28,39,45]. Transduction efficiencies on retrovirus-coated poly-L-lysine were very high (>99%), regardless of the underlying surface chemistry. Notably, biomaterial-mediated gene delivery on the NH2 SAM in the absence of pre-adsorbed extracellular matrix molecules compared favorably to poly-L-lysine-assisted gene transfer.
Figure 5.
Effects of extracellular matrix molecules on biomaterial-mediated gene delivery. The indicated matrix molecule was passively adsorbed to the biomaterial surface prior to virus incubation and subsequent cell seeding. Gene transfer was measured by flow cytometry for eGFP expression at 72 hr post-seeding. * vs. TC, # vs. CH3, † vs. COOH (p<0.001).
DISCUSSION
Biomaterial-mediated gene delivery offers several advantages to conventional methods for genetic engineering of mammalian cells in vitro and in vivo. In particular, this strategy offers the potential to increase the efficiency of gene transfer by co-localizing the cells and viral particles and enhance the safety of gene therapy by avoiding off-target effects of the gene carrier on neighboring cells and tissues. This study characterizes a novel approach to retroviral gene transfer through immobilization of retroviral particles to a biomaterial surface consisting of well-defined, stable self-assembled monolayers. Retrovirus immobilization and transduction efficiency were strongly dependent on biomaterial surface chemistry, with transduction efficiencies of over 90% on the cationic NH2 surface. This method was further characterized as a function of virus-surface incubation time, virus concentration, and cell seeding density. Finally, gene delivery via the NH2 surface was shown to compare favorably with poly-L-lysine- and fibronectin-assisted gene transfer. In summary, these results have established a novel application of SAMs in biomaterial-mediated gene delivery and provided insights into the design of safe and effective gene therapy strategies.
Numerous genetic engineering strategies have been developed that take advantage of the electrostatic interactions between gene carriers, including viruses and plasmid DNA, and molecules to enhance gene delivery, such as poly-L-lysine and polyethylenimine [1,2]. These cationic agents function by reducing the electrostatic repulsion between the anionic lipid bilayers of cells and retroviruses and the phosphate backbone of plasmid DNA [30]. Furthermore, anionic compounds, such as chondroitin sulfate C, may act as polymer bridges by complexing with cationic molecules which subsequently recruit negatively charged cells, virus, and/or plasmid DNA [30]. However, many of these soluble agents are toxic to cells in culture and/or when delivered in vivo [2]. Therefore, it is desirable to incorporate these chemical properties into the design of the biomaterial such that these additional agents are not necessary for gene immobilization. The current study provides insights into the biomaterial properties that mediate retrovirus immobilization and demonstrates that both cationic and anionic surfaces, in this case alkanethiol SAMs, represent promising formats for biomaterial-assisted retroviral transduction. We hypothesize that these charged surfaces function through a similar mechanism as the soluble charged agents described above. The lack of gene transfer on CH3 surfaces may be the result of absence of these electrostatic interactions or the substantial denaturation of adsorbed proteins on this highly hydrophobic surface [39].
The material properties that favor retroviral gene delivery may be incorporated into the design of cell culture supports and subsequently used as a method to genetically engineer cells for basic biological studies or used to increase gene transfer efficiencies for ex vivo gene therapy. Alternatively, retrovirus-coated materials could be directly implanted to enhance the repair of diseased or damaged tissue [3]. For example, gene-activated biomaterials have been used in combination with bone grafts [4,18,22], arterial stents [38,46], and treatments to regulate angiogenesis [5]. Therapeutic retroviral particles have also been directly injected into bone defects to enhance tissue regeneration [47], administered intravenously to correct genetic diseases [48,49], and delivered to tumors to treat brain cancer [43]. Combining these approaches into a single strategy based on biomaterial-mediated retroviral gene delivery may significantly enhance the efficacy of these therapies. Importantly, we have observed similar results of virus immobilization to SAMs with lentiviral supernatants (unpublished results), which are capable of transducing non-dividing cells [44]. Therefore this approach may be applicable to developing therapies for a broad range of cell targets, tissue defects, and diseases.
SAMs have predominantly been used as in vitro model systems to study the effects of surface properties on the interactions of materials with cells and proteins [35]. However, SAMs have recently been used to study in vivo inflammatory responses following implantation [50,51]. These results suggest that SAMs are stable in vivo, at least for short time periods, and may be useful as a therapeutic biomaterial coating in the design of biomedical devices or tissue-engineered constructs. Therefore, methods focusing on SAM-mediated gene delivery may be directly relevant to future gene therapy strategies. The self-assembling properties of SAMs have also been exploited to develop spatially patterned materials at the micro and nano scale [35]. These studies suggest that the immobilization of gene carriers to SAMs may be useful for achieving spatiallyregulated gene transfer, similar to recent approaches for directing neurite extension [52,53] and retrovirus-based microarrays [54,55]. These efforts are essential to engineering tissues of multiple cell types, complex architecture, and morphological features, developing methods for high-throughput functional genomics, and studying basic biological processes, such as intercellular communication.
CONCLUSION
We describe a novel approach to biomaterial-mediated gene delivery via surface immobilization of retroviral particles onto alkanethiol self-assembled monolayers, which impart a permanent genetic modification in target cells. Biomaterial-assisted retroviral gene transfer exhibited strong dependence on the biomaterial surface chemistry as well as viral supernatant and cell culture conditions. This strategy is particularly efficient in engineering mammalian cells and compares favorably with other established methods for enhancing retroviral transduction. This work is significant to establishing safe and effective gene therapy strategies, developing efficient methods for gene delivery, and supporting recent progress in the field of biomaterial-mediated gene transfer.
Acknowledgments
The authors thank T.J. Murphy for generously providing the retroviral vector. This research was funded by the NIH (R01-EB003364), the Georgia Tech/Emory Center NSF Engineering Research Center on Tissue Engineering (EEC-9731643), and a Medtronic Foundation Fellowship and NIH Biotechnology Training Grant (T32-GM08433).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Mol Ther. 2004;10(1):19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Han S, Mahato RI, Sung YK, Kim SW. Development of biomaterials for gene therapy. Mol Ther. 2000;2(4):302–317. doi: 10.1006/mthe.2000.0142. [DOI] [PubMed] [Google Scholar]
- 3.Gersbach CA, Phillips JE, Garcia AJ. Genetic Engineering for Skeletal Regenerative Medicine. Annu Rev Biomed Eng. 2007 doi: 10.1146/annurev.bioeng.9.060906.151949. [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93(12):5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17(6):551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 6.Scherer F, Schillinger U, Putz U, Stemberger A, Plank C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. J Gene Med. 2002;4(6):634–643. doi: 10.1002/jgm.298. [DOI] [PubMed] [Google Scholar]
- 7.Yun YH, Goetz DJ, Yellen P, Chen W. Hyaluronan microspheres for sustained gene delivery and site-specific targeting. Biomaterials. 2004;25(1):147–157. doi: 10.1016/s0142-9612(03)00467-8. [DOI] [PubMed] [Google Scholar]
- 8.Kim A, Checkla DM, Dehazya P, Chen W. Characterization of DNA-hyaluronan matrix for sustained gene transfer. J Control Release. 2003;90(1):81–95. doi: 10.1016/s0168-3659(03)00175-5. [DOI] [PubMed] [Google Scholar]
- 9.Kofron MD, Laurencin CT. Development of a calcium phosphate co-precipitate/poly(lactide-co-glycolide) DNA delivery system: release kinetics and cellular transfection studies. Biomaterials. 2004;25(13):2637–2643. doi: 10.1016/j.biomaterials.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Quick DJ, Anseth KS. Gene delivery in tissue engineering: a photopolymer platform to coencapsulate cells and plasmid DNA. Pharm Res. 2003;20(11):1730–1737. doi: 10.1023/b:pham.0000003368.66471.6a. [DOI] [PubMed] [Google Scholar]
- 11.Shen H, Goldberg E, Saltzman WM. Gene expression and mucosal immune responses after vaginal DNA immunization in mice using a controlled delivery matrix. J Control Release. 2003;86(23):339–348. doi: 10.1016/s0168-3659(02)00354-1. [DOI] [PubMed] [Google Scholar]
- 12.Gonsho A, Irie K, Susaki H, Iwasawa H, Okuno S, Sugawara T. Tissue-targeting ability of saccharide-poly(L-lysine) conjugates. Biol Pharm Bull. 1994;17(2):275–282. doi: 10.1248/bpb.17.275. [DOI] [PubMed] [Google Scholar]
- 13.Boussif O, Lezoualc'h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng J, Manuel WS, Hornsby PJ. Transfection of cells mediated by biodegradable polymer materials with surface-bound polyethyleneimine. Biotechnol Prog. 2000;16(2):254–257. doi: 10.1021/bp990150h. [DOI] [PubMed] [Google Scholar]
- 15.Cohen-Sacks H, Elazar V, Gao J, Golomb A, Adwan H, Korchov N, et al. Delivery and expression of pDNA embedded in collagen matrices. J Control Release. 2004;95(2):309–320. doi: 10.1016/j.jconrel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 16.MacLaughlin FC, Mumper RJ, Wang J, Tagliaferri JM, Gill I, Hinchcliffe M, et al. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J Control Release. 1998;56(13):259–272. doi: 10.1016/s0168-3659(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 17.Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR., Jr Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc Natl Acad Sci U S A. 1996;93(10):4897–4902. doi: 10.1073/pnas.93.10.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schek RM, Hollister SJ, Krebsbach PH. Delivery and protection of adenoviruses using biocompatible hydrogels for localized gene therapy. Mol Ther. 2004;9(1):130–138. doi: 10.1016/j.ymthe.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Sailaja G, HogenEsch H, North A, Hays J, Mittal SK. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002;9(24):1722–1729. doi: 10.1038/sj.gt.3301858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beer SJ, Matthews CB, Stein CS, Ross BD, Hilfinger JM, Davidson BL. Poly (lactic-glycolic) acid copolymer encapsulation of recombinant adenovirus reduces immunogenicity in vivo. Gene Ther. 1998;5(6):740–746. doi: 10.1038/sj.gt.3300647. [DOI] [PubMed] [Google Scholar]
- 21.Matthews C, Jenkins G, Hilfinger J, Davidson B. Poly-L-lysine improves gene transfer with adenovirus formulated in PLGA microspheres. Gene Ther. 1999;6(9):1558–1564. doi: 10.1038/sj.gt.3300978. [DOI] [PubMed] [Google Scholar]
- 22.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11(3):291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2(8):876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 24.Hanenberg H, Hashino K, Konishi H, Hock RA, Kato I, Williams DA. Optimization of fibronectin-assisted retroviral gene transfer into human CD34+ hematopoietic cells. Hum Gene Ther. 1997;8(18):2193–2206. doi: 10.1089/hum.1997.8.18-2193. [DOI] [PubMed] [Google Scholar]
- 25.Bajaj B, Lei P, Andreadis ST. High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol Prog. 2001;17(4):587–596. doi: 10.1021/bp010039n. [DOI] [PubMed] [Google Scholar]
- 26.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10(3):785–798. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A. 2005;102(17):5953–5957. doi: 10.1073/pnas.0407356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan MA, Gersbach CA, Michael KE, Keselowsky BG, Garcia AJ. Myoblast proliferation and differentiation on fibronectin-coated self assembled monolayers presenting different surface chemistries. Biomaterials. 2005;26(22):4523–4531. doi: 10.1016/j.biomaterials.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Themis M, Forbes SJ, Chan L, Cooper RG, Etheridge CJ, Miller AD, et al. Enhanced in vitro and in vivo gene delivery using cationic agent complexed retrovirus vectors. Gene Ther. 1998;5(9):1180–1186. doi: 10.1038/sj.gt.3300715. [DOI] [PubMed] [Google Scholar]
- 30.Le Doux JM, Landazuri N, Yarmush ML, Morgan JR. Complexation of retrovirus with cationic and anionic polymers increases the efficiency of gene transfer. Hum Gene Ther. 2001;12(13):1611–1621. doi: 10.1089/10430340152528110. [DOI] [PubMed] [Google Scholar]
- 31.Landazuri N, Le Doux JM. Complexation of retroviruses with charged polymers enhances gene transfer by increasing the rate that viruses are delivered to cells. J Gene Med. 2004;6(12):1304–1319. doi: 10.1002/jgm.618. [DOI] [PubMed] [Google Scholar]
- 32.Hennemann B, Chuo JY, Schley PD, Lambie K, Humphries RK, Eaves CJ. High-efficiency retroviral transduction of mammalian cells on positively charged surfaces. Hum Gene Ther. 2000;11(1):43–51. doi: 10.1089/10430340050016148. [DOI] [PubMed] [Google Scholar]
- 33.Hennemann B, Oh IH, Chuo JY, Kalberer CP, Schley PD, Rose-John S, et al. Efficient retrovirus-mediated gene transfer to transplantable human bone marrow cells in the absence of fibronectin. Blood. 2000;96(7):2432–2439. [PubMed] [Google Scholar]
- 34.Kuhlcke K, Fehse B, Schilz A, Loges S, Lindemann C, Ayuk F, et al. Highly efficient retroviral gene transfer based on centrifugation-mediated vector preloading of tissue culture vessels. Mol Ther. 2002;5(4):473–478. doi: 10.1006/mthe.2002.0566. [DOI] [PubMed] [Google Scholar]
- 35.Whitesides GM, Kriebel JK, Love JC. Molecular engineering of surfaces using self-assembled monolayers. Sci Prog. 2005;88(Pt 1):17–48. doi: 10.3184/003685005783238462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pannier AK, Anderson BC, Shea LD. Substrate-mediated delivery from self-assembled monolayers: effect of surface ionization, hydrophilicity, and patterning. Acta Biomater. 2005;1(5):511–522. doi: 10.1016/j.actbio.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi F, Kato K, Iwata H. Micropatterned, self-assembled monolayers for fabrication of transfected cell microarrays. Biochim Biophys Acta. 2004;1672(3):138–147. doi: 10.1016/j.bbagen.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Yamauchi F, Koyamatsu Y, Kato K, Iwata H. Layer-by-layer assembly of cationic lipid and plasmid DNA onto gold surface for stent-assisted gene transfer. Biomaterials. 2006;27(18):3497–3504. doi: 10.1016/j.biomaterials.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion. J Biomed Mater Res A. 2003;66(2):247–259. doi: 10.1002/jbm.a.10537. [DOI] [PubMed] [Google Scholar]
- 40.Murphy TJ, Pavlath GK, Wang X, Boss V, Abbott KL, Robida AM et al. Retroviral vectors applied to gene regulation studies. Methods Enzymol. 2002:345539–551. doi: 10.1016/s0076-6879(02)45045-8. [DOI] [PubMed] [Google Scholar]
- 41.Byers BA, Pavlath GK, Murphy TJ, Karsenty G, Garcia AJ. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblastspecific transcription factor Runx2/Cbfal. J Bone Miner Res. 2002;17(11):1931–1944. doi: 10.1359/jbmr.2002.17.11.1931. [DOI] [PubMed] [Google Scholar]
- 42.Le Doux JM, Davis HE, Morgan JR, Yarmush ML. Kinetics of retrovirus production and decay. Biotechnol Bioeng. 1999;63(6):654–662. [PubMed] [Google Scholar]
- 43.Galipeau J, Li H, Paquin A, Sicilia F, Karpati G, Nalbantoglu J. Vesicular stomatitis virus G pseudotyped retrovector mediates effective in vivo suicide gene delivery in experimental brain cancer. Cancer Res. 1999;59(10):2384–2394. [PubMed] [Google Scholar]
- 44.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272(5259):263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 45.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25(28):5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 46.Klugherz BD, Jones PL, Cui X, Chen W, Meneveau NF, DeFelice S, et al. Gene delivery from a DNA controlled-release stent in porcine coronary arteries. Nat Biotechnol. 2000;18(11):1181–1184. doi: 10.1038/81176. [DOI] [PubMed] [Google Scholar]
- 47.Rundle CH, Miyakoshi N, Kasukawa Y, Chen ST, Sheng MH, Wergedal JE, et al. In vivo bone formation in fracture repair induced by direct retroviral-based gene therapy with bone morphogenetic protein-4. Bone. 2003;32(6):591–601. doi: 10.1016/s8756-3282(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 48.Xu L, Gao C, Sands MS, Cai SR, Nichols TC, Bellinger DA, et al. Neonatal or hepatocyte growth factor-potentiated adult gene therapy with a retroviral vector results in therapeutic levels of canine factor IX for hemophilia B. Blood. 2003;101(10):3924–3932. doi: 10.1182/blood-2002-10-3050. [DOI] [PubMed] [Google Scholar]
- 49.Xu L, O'Malley T, Sands MS, Wang B, Meyerrose T, Haskins ME, et al. In vivo transduction of hematopoietic stem cells after neonatal intravenous injection of an amphotropic retroviral vector in mice. Mol Ther. 2004;10(1):37–44. doi: 10.1016/j.ymthe.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Barbosa JN, Barbosa MA, Aguas AP. Inflammatory responses and cell adhesion to self-assembled monolayers of alkanethiolates on gold. Biomaterials. 2004;25(13):2557–2563. doi: 10.1016/j.biomaterials.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 51.Barbosa JN, Madureira P, Barbosa MA, Aguas AP. The influence of functional groups of self-assembled monolayers on fibrous capsule formation and cell recruitment. J Biomed Mater Res A. 2006;76(4):737–743. doi: 10.1002/jbm.a.30602. [DOI] [PubMed] [Google Scholar]
- 52.Houchin-Ray T, Whittlesey KJ, Shea LD. Spatially patterned gene delivery for localized neuron survival and neurite extension. Mol Ther. 2007;15(4):705–712. doi: 10.1038/mt.sj.6300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houchin-Ray T, Swift LA, Jang JH, Shea LD. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials. 2007;28(16):2603–2611. doi: 10.1016/j.biomaterials.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey SN, Ali SM, Carpenter AE, Higgins CO, Sabatini DM. Microarrays of lentiviruses for gene function screens in immortalized and primary cells. Nat Methods. 2006;3(2):117–122. doi: 10.1038/nmeth848. [DOI] [PubMed] [Google Scholar]
- 55.Carbone R, Giorgetti L, Zanardi A, Marangi I, Chierici E, Bongiorno G, et al. Retroviral microarray-based platform on nanostructured TiO(2) for functional genomics and drug discovery. Biomaterials. 2007;28(13):2244–2253. doi: 10.1016/j.biomaterials.2006.12.026. [DOI] [PubMed] [Google Scholar]