Abstract
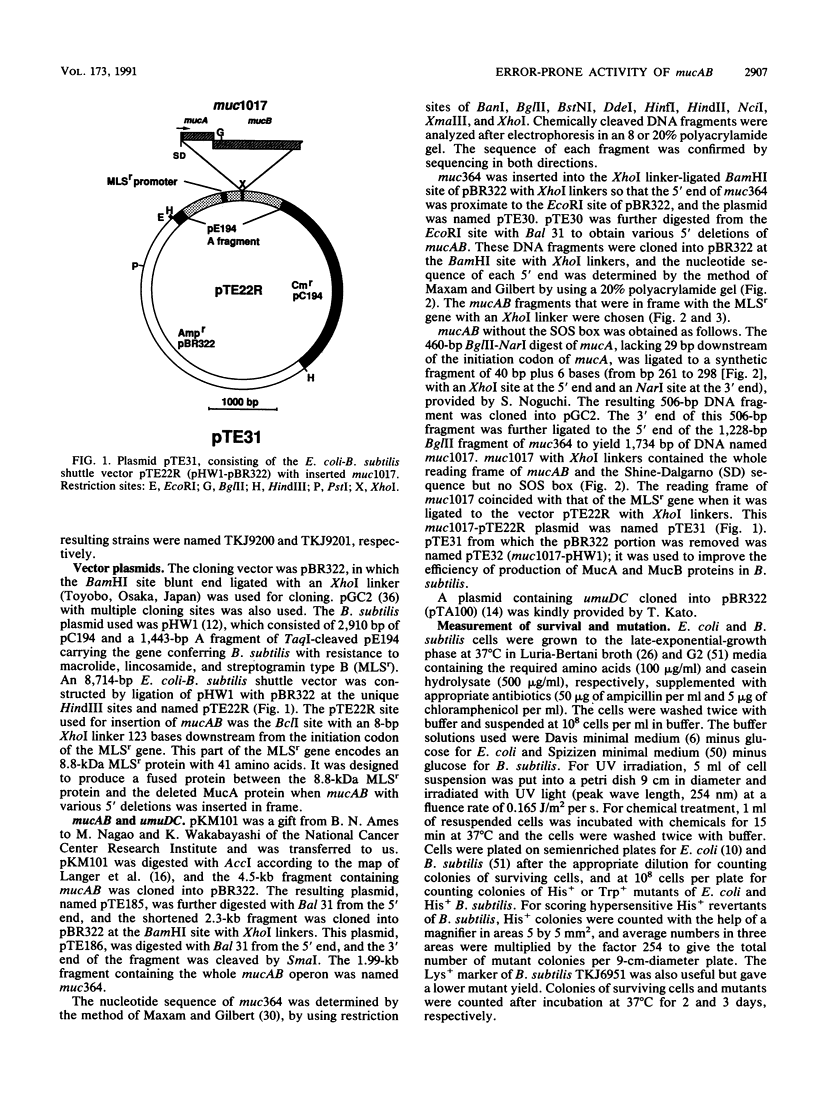
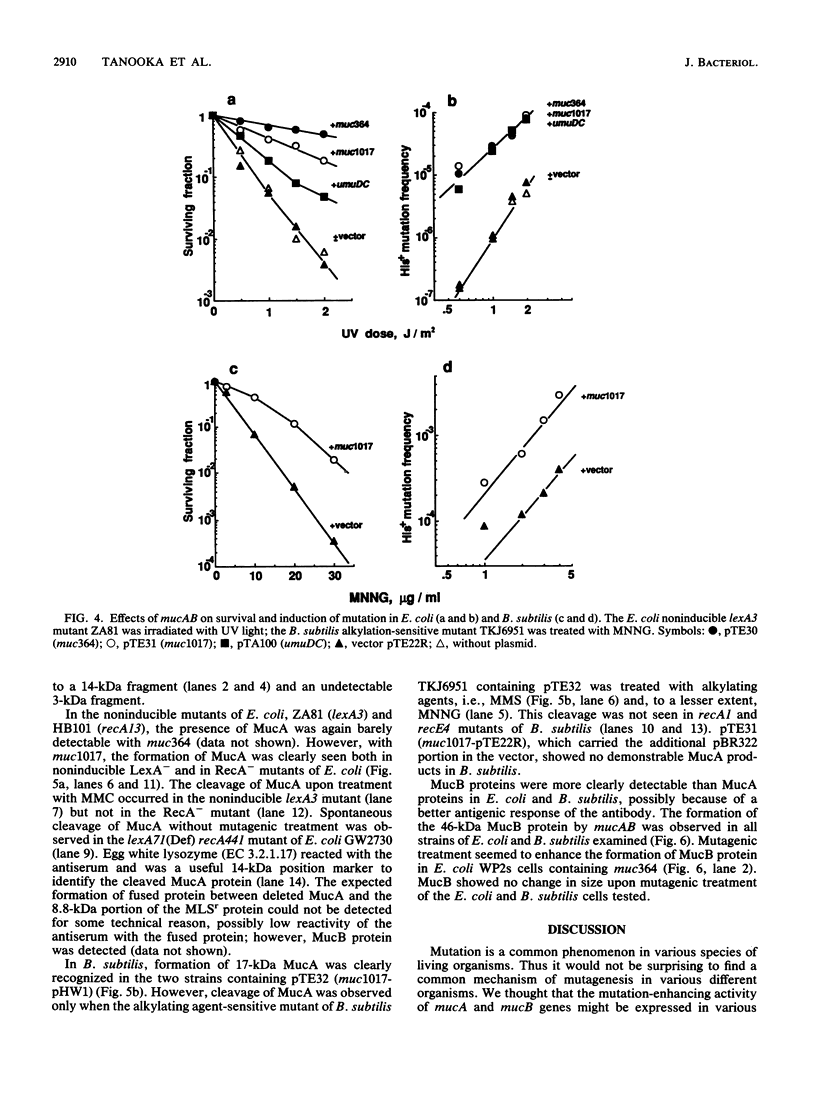
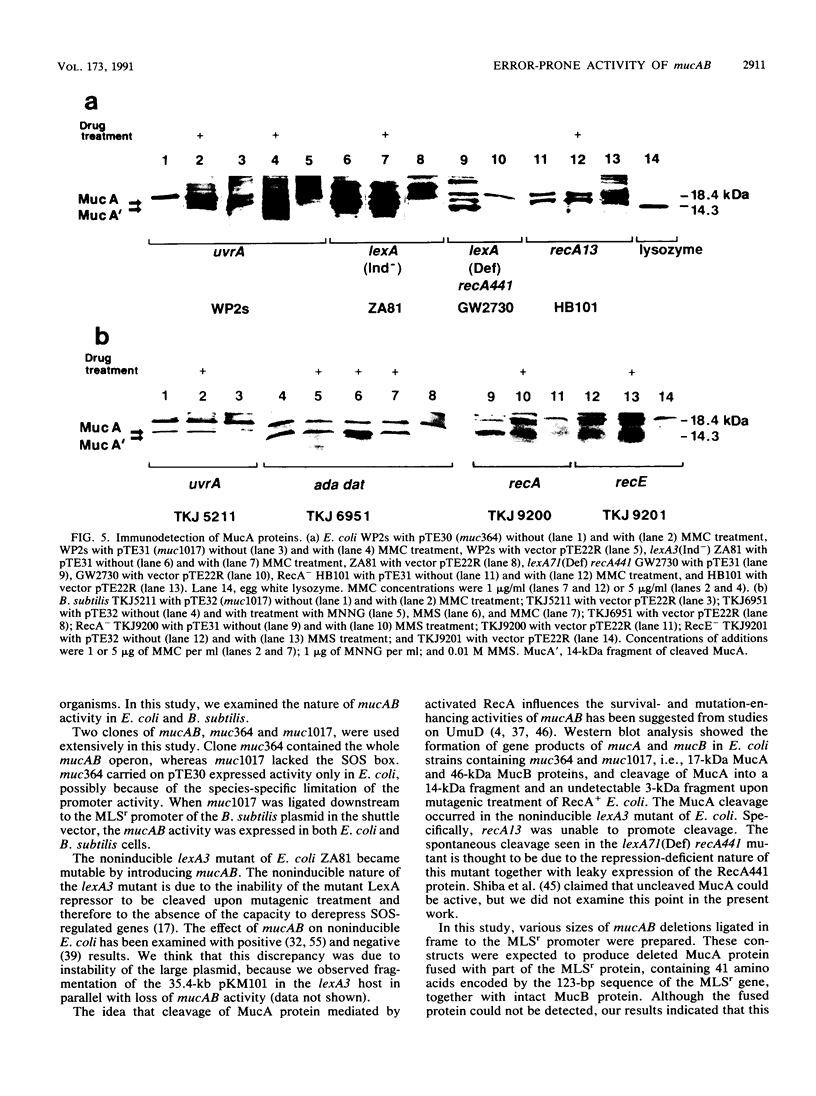
Enterobacterial plasmid genes mucAB, which possess error-prone repair activity, were cloned and sequenced independently of a sequence previously determined (K.L. Perry, S.J. Elledge, B.B. Mitchell, L. Marsh, and G.C. Walker, Proc. Natl. Acad. Sci. USA 82:4331-4335, 1985). The survival- and mutation-enhancing activities of mucAB ligated to the MLSr promoter of a Bacillus subtilis plasmid in the shuttle vector pTE22R were expressed in B. subtilis as well as in Escherichia coli after mutagenic treatment. mucAB fragments with 5' deletions of various lengths up to the base sequence encoding Ala-26-Gly-27, the putative RecA-mediated cleavage site of the MucA protein, showed mutation-enhancing activity for noninducible lexA3 E. coli when ligated to the MLSr promoter in frame. This activity was lost by extending the deletion downstream. The formations of MucA and MucB proteins in B. subtilis and E. coli were demonstrated by Western blot (immunoblot) analysis. MucA cleavage in Rec+ B. subtilis was observed only after treatment with an alkylating agent and was not observed in RecA- and RecE- strains, whereas in E. coli cleavage was observed in Rec+ cells after treatment with either mitomycin C or an alkylating agent but was not detected in RecA- cells. Common activity of B. subtilis Rec and E. coli RecA in the induction of mutants is suggested.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balganesh M., Setlow J. K. Genes from plasmid pKM101 in Haemophilus influenzae: separation of functions of mucA and mucB. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7753–7756. doi: 10.1073/pnas.82.22.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P., Rothwell M. A., Green M. H. Repair-deficient bacterial strains suitable for mutagenicity screening: tests with the fungicide captain. Chem Biol Interact. 1972 Jul;5(2):77–84. doi: 10.1016/0009-2797(72)90034-8. [DOI] [PubMed] [Google Scholar]
- Burckhardt S. E., Woodgate R., Scheuermann R. H., Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification, and cleavage by RecA. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Drabble W. T., Stocker B. A. R (transmissible drug-resistance) factors in Salmonella typhimurium: pattern of transduction by phage P22 and ultraviolet-protection effect. J Gen Microbiol. 1968 Aug;53(1):109–123. doi: 10.1099/00221287-53-1-109. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Cirigliano C. Genetic characterization of recombination-deficient mutants of Bacillus subtilis. J Bacteriol. 1974 Feb;117(2):488–493. doi: 10.1128/jb.117.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. H., Muriel W. J. Mutagen testing using TRP+ reversion in Escherichia coli. Mutat Res. 1976 Feb;38(1):3–32. doi: 10.1016/0165-1161(76)90076-5. [DOI] [PubMed] [Google Scholar]
- Horii T., Ogawa T., Nakatani T., Hase T., Matsubara H., Ogawa H. Regulation of SOS functions: purification of E. coli LexA protein and determination of its specific site cleaved by the RecA protein. Cell. 1981 Dec;27(3 Pt 2):515–522. doi: 10.1016/0092-8674(81)90393-7. [DOI] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y., Akaboshi E., Shinagawa H., Horii T., Ogawa H., Kato T. Structural analysis of the umu operon required for inducible mutagenesis in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4336–4340. doi: 10.1073/pnas.82.13.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. H., Elledge S. J., Walker G. C. Isolation and characterization of Tn5 insertion mutations in the lexA gene of Escherichia coli. J Bacteriol. 1983 Mar;153(3):1368–1378. doi: 10.1128/jb.153.3.1368-1378.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer P. J., Shanabruch W. G., Walker G. C. Functional organization of plasmid pKM101. J Bacteriol. 1981 Mar;145(3):1310–1316. doi: 10.1128/jb.145.3.1310-1316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W., Yanisch-Perron C. R. Purified lexA protein is a repressor of the recA and lexA genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4199–4203. doi: 10.1073/pnas.78.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodwick D., Owen D., Strike P. DNA sequence analysis of the imp UV protection and mutation operon of the plasmid TP110: identification of a third gene. Nucleic Acids Res. 1990 Sep 11;18(17):5045–5050. doi: 10.1093/nar/18.17.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love P. E., Lyle M. J., Yasbin R. E. DNA-damage-inducible (din) loci are transcriptionally activated in competent Bacillus subtilis. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6201–6205. doi: 10.1073/pnas.82.18.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Love P. E., Yasbin R. E., Roberts J. W. SOS-like induction in Bacillus subtilis: induction of the RecA protein analog and a damage-inducible operon by DNA damage in Rec+ and DNA repair-deficient strains. J Bacteriol. 1988 Apr;170(4):1467–1474. doi: 10.1128/jb.170.4.1467-1474.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett C. M., Jr, Roberts J. W. Purification of a RecA protein analogue from Bacillus subtilis. J Biol Chem. 1985 Mar 25;260(6):3305–3313. [PubMed] [Google Scholar]
- MacPhee D. G. Effect of an R factor on resistance of Salmonella typhimurium to radiation and chemical treatment. Mutat Res. 1972 Apr;14(4):450–453. doi: 10.1016/0027-5107(72)90146-7. [DOI] [PubMed] [Google Scholar]
- MacPhee D. G. Effect of rec mutations on the ultraviolet protecting and mutation-enhancing properties of the plasmid R-Utrecht in Salmonella typhimurium. Mutat Res. 1973 Sep;19(3):357–359. doi: 10.1016/0027-5107(73)90237-6. [DOI] [PubMed] [Google Scholar]
- Marcucci L., Gigliani F., Battaglia P. A., Bosi R., Sporeno E., Elli R. Cellular response to DNA damage is enhanced by the pR plasmid in mouse cells and in Escherichia coli. Mol Cell Biol. 1986 Feb;6(2):586–592. doi: 10.1128/mcb.6.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Walker G. C. Cold sensitivity induced by overproduction of UmuDC in Escherichia coli. J Bacteriol. 1985 Apr;162(1):155–161. doi: 10.1128/jb.162.1.155-161.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L., Walker G. C. New phenotypes associated with mucAB: alteration of a MucA sequence homologous to the LexA cleavage site. J Bacteriol. 1987 May;169(5):1818–1823. doi: 10.1128/jb.169.5.1818-1823.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti-Bragadin C., Babudri N., Samer L. Expression of the plasmid pKM101--determined DNA repair system in recA- and lex- strains of Escherichia coli. Mol Gen Genet. 1976 Jun 15;145(3):303–306. doi: 10.1007/BF00325827. [DOI] [PubMed] [Google Scholar]
- Morohoshi F., Munakata N. Isolation of a Bacillus subtilis mutant defective in constitutive O6-alkylguanine-DNA alkyltransferase. Mutat Res. 1990 Jan;235(1):15–23. doi: 10.1016/0921-8777(90)90018-z. [DOI] [PubMed] [Google Scholar]
- Mortelmans K. E., Stocker B. A. Ultraviolet light protection, enhancement of ultraviolet light mutagenesis, and mutator effect of plasmid R46 in Salmonella typhimurium. J Bacteriol. 1976 Oct;128(1):271–282. doi: 10.1128/jb.128.1.271-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Lerman L. S., Maniatis T. A general method for saturation mutagenesis of cloned DNA fragments. Science. 1985 Jul 19;229(4710):242–247. doi: 10.1126/science.2990046. [DOI] [PubMed] [Google Scholar]
- Nohmi T., Battista J. R., Dodson L. A., Walker G. C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohmi T., Hakura A., Nakai Y., Watanabe M., Murayama S. Y., Sofuni T. Salmonella typhimurium has two homologous but different umuDC operons: cloning of a new umuDC-like operon (samAB) present in a 60-megadalton cryptic plasmid of S. typhimurium. J Bacteriol. 1991 Feb;173(3):1051–1063. doi: 10.1128/jb.173.3.1051-1063.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T., Nishioka H. Effect of pKM101 plasmid on lethal and mutagenic damage in UV-irradiated E. coli strains. Mutat Res. 1987 Jan;183(1):39–44. doi: 10.1016/0167-8817(87)90043-5. [DOI] [PubMed] [Google Scholar]
- Okubo S., Yanagida T. Isolation of a suppressor mutant in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):1187–1188. doi: 10.1128/jb.95.3.1187-1188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry K. L., Elledge S. J., Mitchell B. B., Marsh L., Walker G. C. umuDC and mucAB operons whose products are required for UV light- and chemical-induced mutagenesis: UmuD, MucA, and LexA proteins share homology. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4331–4335. doi: 10.1073/pnas.82.13.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A. A., Nestmann E. R., Iyer V. N. Introduction of the plasmid pKM101-associated muc genes into Saccharomyces cerevisiae. Mutat Res. 1984 May-Jun;131(5-6):197–204. doi: 10.1016/0167-8817(84)90025-7. [DOI] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Shiba T., Iwasaki H., Nakata A., Shinagawa H. Proteolytic processing of MucA protein in SOS mutagenesis: both processed and unprocessed MucA may be active in the mutagenesis. Mol Gen Genet. 1990 Nov;224(2):169–176. doi: 10.1007/BF00271549. [DOI] [PubMed] [Google Scholar]
- Shinagawa H., Iwasaki H., Kato T., Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slilaty S. N., Little J. W. Lysine-156 and serine-119 are required for LexA repressor cleavage: a possible mechanism. Proc Natl Acad Sci U S A. 1987 Jun;84(12):3987–3991. doi: 10.1073/pnas.84.12.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. M., Koch W. H., Franklin S. B., Foster P. L., Cebula T. A., Eisenstadt E. Sequence analysis and mapping of the Salmonella typhimurium LT2 umuDC operon. J Bacteriol. 1990 Sep;172(9):4964–4978. doi: 10.1128/jb.172.9.4964-4978.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spikes D., Setlow J. K. A plasmid carrying mucA and mucB genes from pKM101 in Haemophilus influenzae and Escherichia coli. J Bacteriol. 1989 Oct;171(10):5753–5755. doi: 10.1128/jb.171.10.5753-5755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanooka H. Development and applications of Bacillus subtilis test systems for mutagens, involving DNA-repair deficiency and suppressible auxotrophic mutations. Mutat Res. 1977 Jan;42(1):19–31. doi: 10.1016/s0027-5107(77)80004-3. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Crowne H. M., Pidsley S. C., Sedgwick S. G. Structural characterization of the Salmonella typhimurium LT2 umu operon. J Bacteriol. 1990 Sep;172(9):4979–4987. doi: 10.1128/jb.172.9.4979-4987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosu M., Tanooka H. Transformation of mouse BALB 3T3 cells by enterobacterial plasmid misrepair gene mucAB. Mol Cell Biol. 1990 Oct;10(10):5359–5364. doi: 10.1128/mcb.10.10.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleh N. S., Stocker B. A. Effect of host lex, recA, recF, and uvrD genotypes on the ultraviolet light-protecting and related properties of plasmid R46 in Escherichia coli. J Bacteriol. 1979 Feb;137(2):830–838. doi: 10.1128/jb.137.2.830-838.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M., George D. L. Ultraviolet mutagenesis in polA and UvrA polA derivatives of Escherichia coli B-R: evidence for an inducible error-prone repair system. Genetics. 1973 Apr;73(Suppl):91–10. [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgate R., Rajagopalan M., Lu C., Echols H. UmuC mutagenesis protein of Escherichia coli: purification and interaction with UmuD and UmuD'. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7301–7305. doi: 10.1073/pnas.86.19.7301. [DOI] [PMC free article] [PubMed] [Google Scholar]