Abstract
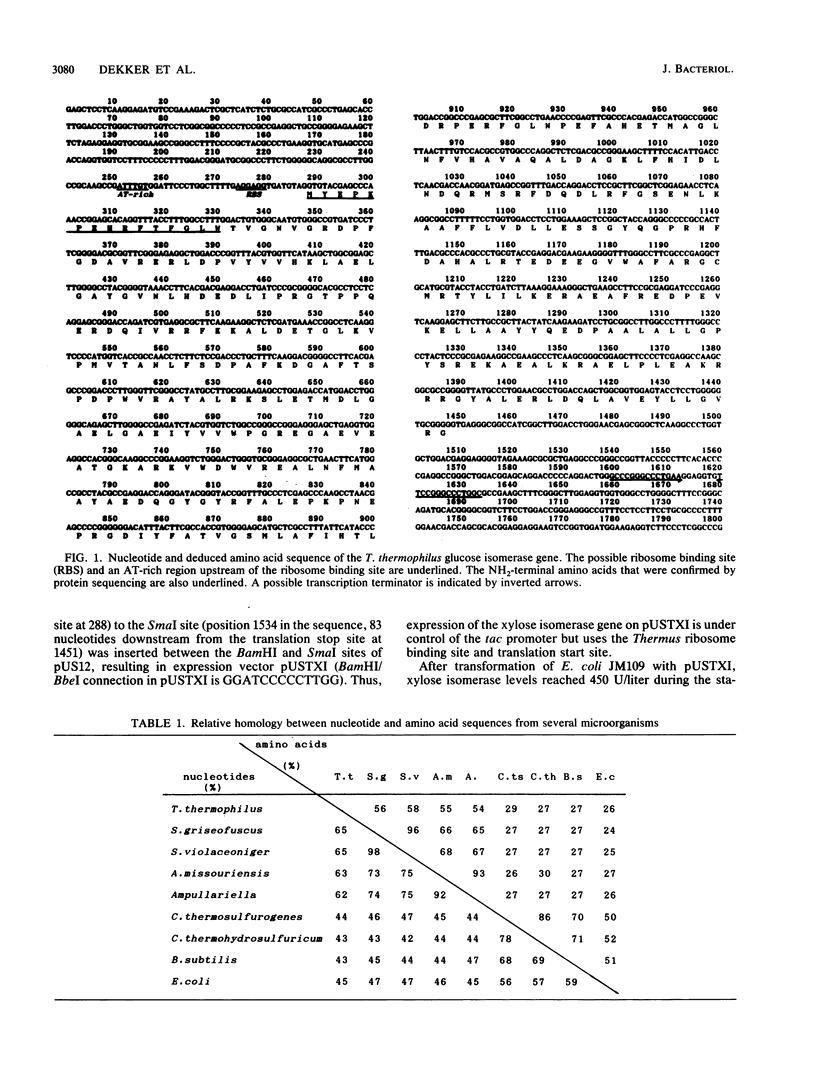
The xylose isomerase gene from the thermophile Thermus thermophilus was cloned by using a fragment of the Streptomyces griseofuscus gene as a probe. The complete nucleotide sequence of the gene was determined. T. thermophilus is the most thermophilic organism from which a xylose isomerase gene has been cloned and characterized. The gene codes for a polypeptide of 387 amino acids with a molecular weight of 44,000. The Thermus xylose isomerase is considerably more thermostable than other described xylose isomerases. Production of the enzyme in Escherichia coli, by using the tac promoter, increases the xylose isomerase yield 45-fold compared with production in T. thermophilus. Moreover, the enzyme from E. coli can be purified 20-fold by simply heating the cell extract at 85 degrees C for 10 min. The characteristics of the enzyme made in E. coli are the same as those of enzyme made in T. thermophilus. Comparison of the Thermus xylose isomerase amino acid sequence with xylose isomerase sequences from other organisms showed that amino acids involved in substrate binding and isomerization are well conserved. Analysis of amino acid substitutions that distinguish the Thermus xylose isomerase from other thermostable xylose isomerases suggests that the further increase in thermostability in T. thermophilus is due to substitution of amino acids which react during irreversible inactivation and results also from increased hydrophobicity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern T. J., Klibanov A. M. The mechanisms of irreversible enzyme inactivation at 100C. Science. 1985 Jun 14;228(4705):1280–1284. doi: 10.1126/science.4001942. [DOI] [PubMed] [Google Scholar]
- Amore R., Hollenberg C. P. Xylose isomerase from Actinoplanes missouriensis: primary structure of the gene and the protein. Nucleic Acids Res. 1989 Sep 25;17(18):7515–7515. doi: 10.1093/nar/17.18.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Rossman M. G., Grau U. M., Zuber H., Frank G., Tratschin J. D. Thermal stability and protein structure. Biochemistry. 1979 Dec 11;18(25):5698–5703. doi: 10.1021/bi00592a028. [DOI] [PubMed] [Google Scholar]
- Carrell H. L., Glusker J. P., Burger V., Manfre F., Tritsch D., Biellmann J. F. X-ray analysis of D-xylose isomerase at 1.9 A: native enzyme in complex with substrate and with a mechanism-designed inactivator. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4440–4444. doi: 10.1073/pnas.86.12.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collyer C. A., Blow D. M. Observations of reaction intermediates and the mechanism of aldose-ketose interconversion by D-xylose isomerase. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1362–1366. doi: 10.1073/pnas.87.4.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collyer C. A., Henrick K., Blow D. M. Mechanism for aldose-ketose interconversion by D-xylose isomerase involving ring opening followed by a 1,2-hydride shift. J Mol Biol. 1990 Mar 5;212(1):211–235. doi: 10.1016/0022-2836(90)90316-E. [DOI] [PubMed] [Google Scholar]
- Dekker K., Yamagata H., Sakaguchi K., Udaka S. Xylose (glucose) isomerase gene from the thermophile Clostridium thermohydrosulfuricum; cloning, sequencing, and expression in Escherichia coli. Agric Biol Chem. 1991 Jan;55(1):221–227. [PubMed] [Google Scholar]
- Drocourt D., Bejar S., Calmels T., Reynes J. P., Tiraby G. Nucleotide sequence of the xylose isomerase gene from Streptomyces violaceoniger. Nucleic Acids Res. 1988 Oct 11;16(19):9337–9337. doi: 10.1093/nar/16.19.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Kikuchi T., Itoh Y., Kasumi T., Fukazawa C. Molecular cloning of the xylA gene encoding xylose isomerase from Streptomyces griseofuscus S-41: primary structure of the gene and its product. Agric Biol Chem. 1990 Sep;54(9):2469–2472. [PubMed] [Google Scholar]
- Kushiro M., Shimizu M., Tomita K. Molecular cloning and sequence determination of the tuf gene coding for the elongation factor Tu of Thermus thermophilus HB8. Eur J Biochem. 1987 Dec 30;170(1-2):93–98. doi: 10.1111/j.1432-1033.1987.tb13671.x. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Bagdasarian M., Meng M. H., Zeikus J. G. Catalytic mechanism of xylose (glucose) isomerase from Clostridium thermosulfurogenes. Characterization of the structural gene and function of active site histidine. J Biol Chem. 1990 Nov 5;265(31):19082–19090. [PubMed] [Google Scholar]
- Lehmacher A., Bisswanger H. Comparative kinetics of D-xylose and D-glucose isomerase activities of the D-xylose isomerase from Thermus aquaticus HB8. Biol Chem Hoppe Seyler. 1990 Jun;371(6):527–536. doi: 10.1515/bchm3.1990.371.1.527. [DOI] [PubMed] [Google Scholar]
- Matthews B. W., Nicholson H., Becktel W. J. Enhanced protein thermostability from site-directed mutations that decrease the entropy of unfolding. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6663–6667. doi: 10.1073/pnas.84.19.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menéndez-Arias L., Argos P. Engineering protein thermal stability. Sequence statistics point to residue substitutions in alpha-helices. J Mol Biol. 1989 Mar 20;206(2):397–406. doi: 10.1016/0022-2836(89)90488-9. [DOI] [PubMed] [Google Scholar]
- Nicholls D. J., Sundaram T. K., Atkinson T., Minton N. P. Cloning and nucleotide sequences of the mdh and sucD genes from Thermus aquaticus B. FEMS Microbiol Lett. 1990 Jun 15;58(1):7–14. doi: 10.1016/0378-1097(90)90093-6. [DOI] [PubMed] [Google Scholar]
- Nishiyama M., Matsubara N., Yamamoto K., Iijima S., Uozumi T., Beppu T. Nucleotide sequence of the malate dehydrogenase gene of Thermus flavus and its mutation directing an increase in enzyme activity. J Biol Chem. 1986 Oct 25;261(30):14178–14183. [PubMed] [Google Scholar]
- Rey F., Jenkins J., Janin J., Lasters I., Alard P., Claessens M., Matthyssens G., Wodak S. Structural analysis of the 2.8 A model of Xylose isomerase from Actinoplanes missouriensis. Proteins. 1988;4(3):165–172. doi: 10.1002/prot.340040303. [DOI] [PubMed] [Google Scholar]
- Saari G. C., Kumar A. A., Kawasaki G. H., Insley M. Y., O'Hara P. J. Sequence of the Ampullariella sp. strain 3876 gene coding for xylose isomerase. J Bacteriol. 1987 Feb;169(2):612–618. doi: 10.1128/jb.169.2.612-618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg G. D., Sarthy A., Larson A. E., Backer M. P., Crabb J. W., Lidstrom M., Hall B. D., Furlong C. E. Xylose isomerase from Escherichia coli. Characterization of the protein and the structural gene. J Biol Chem. 1984 Jun 10;259(11):6826–6832. [PubMed] [Google Scholar]
- Tanaka T., Kawano N., Oshima T. Cloning of 3-isopropylmalate dehydrogenase gene of an extreme thermophile and partial purification of the gene product. J Biochem. 1981 Feb;89(2):677–682. doi: 10.1093/oxfordjournals.jbchem.a133245. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wilhelm M., Hollenberg C. P. Nucleotide sequence of the Bacillus subtilis xylose isomerase gene: extensive homology between the Bacillus and Escherichia coli enzyme. Nucleic Acids Res. 1985 Aug 12;13(15):5717–5722. doi: 10.1093/nar/13.15.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]


