Abstract
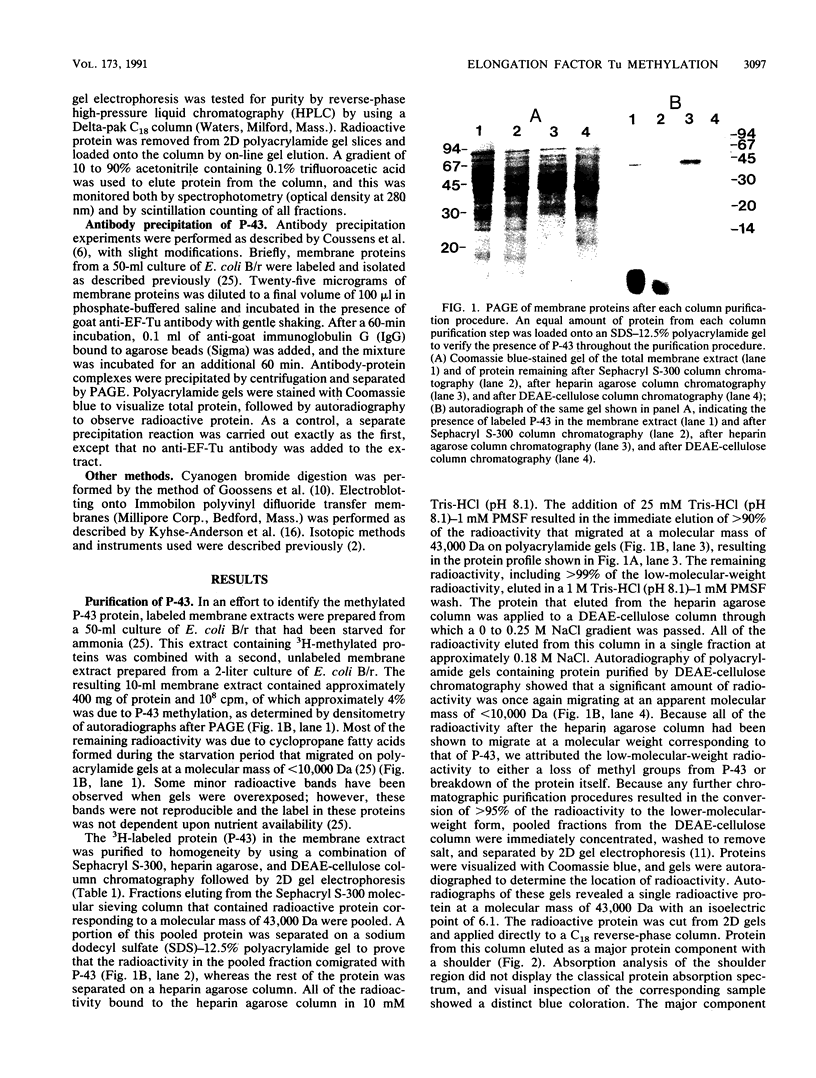
It has been shown previously that starvation of a mid-logarithmic-phase culture of Escherichia coli B/r for an essential nutrient results in the methylation of a membrane-associated protein (P-43) (C. C. Young and R. W. Bernlohr, J. Bacteriol. 172:5147-5153, 1990). In this communication, the purification of P-43 and sequence analysis of cyanogen bromide-generated peptide fragments identified P-43 as elongation factor Tu (EF-Tu). This was confirmed by the ability of anti-EF-Tu antibody to precipitate P-43. We propose that the nutrient-dependent methylation of EF-Tu may be involved in the regulation of growth, possibly as a principal component of an unidentified signal transduction pathway in bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballinger D. G., Pardue M. L. The control of protein synthesis during heat shock in Drosophila cells involves altered polypeptide elongation rates. Cell. 1983 May;33(1):103–113. doi: 10.1016/0092-8674(83)90339-2. [DOI] [PubMed] [Google Scholar]
- Bernlohr R. W., Saha A. L., Young C. C., Toth B. R., Golden K. J. Nutrient-stimulated methylation of a membrane protein in Bacillus licheniformis. J Bacteriol. 1988 Sep;170(9):4113–4118. doi: 10.1128/jb.170.9.4113-4118.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool R. H., Jensen M., Jonák J., Clark B. F., Parmeggiani A. Substitution of proline 82 by threonine induces autophosphorylating activity in GTP-binding domain of elongation factor Tu. J Biol Chem. 1990 Apr 25;265(12):6744–6749. [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Heinze J. E., Galliers E. M. Partial purine deprivation causes sporulation of Bacillus subtilis in the presence of excess ammonia, glucose and phosphate. J Gen Microbiol. 1979 Nov;115(1):193–205. doi: 10.1099/00221287-115-1-193. [DOI] [PubMed] [Google Scholar]
- Golden K. J., Bernlohr R. W. Defects in the nutrient-dependent methylation of a membrane-associated protein in spo mutants of Bacillus subtilis. Mol Gen Genet. 1989 Dec;220(1):1–7. doi: 10.1007/BF00260847. [DOI] [PubMed] [Google Scholar]
- Goossens W., Préaux G., Lontie R. Cleavage of bovine serum albumin with cyanogen bromide and alignment of the fragments. Biochimie. 1973;55(10):1199–1207. doi: 10.1016/s0300-9084(74)80324-x. [DOI] [PubMed] [Google Scholar]
- Hall J. C., Killian G. J. Two-dimensional gel electrophoretic analysis of rat sperm membrane interaction with cauda epididymal fluid. J Androl. 1989 Jan-Feb;10(1):64–76. doi: 10.1002/j.1939-4640.1989.tb00063.x. [DOI] [PubMed] [Google Scholar]
- Halliday K. R. Regional homology in GTP-binding proto-oncogene products and elongation factors. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):435–448. [PubMed] [Google Scholar]
- Haseltine W. A. In vitro transcription of Escherichia coli ribosomal RNA genes. Nature. 1972 Feb 11;235(5337):329–333. doi: 10.1038/235329a0. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. Abundance and membrane association of elongation factor Tu in E. coli. Nature. 1976 May 6;261(5555):23–26. doi: 10.1038/261023a0. [DOI] [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984 Dec;10(3-4):203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- L'Italien J. J., Laursen R. A. Location of the site of methylation in elongation factor Tu. FEBS Lett. 1979 Nov 15;107(2):359–362. doi: 10.1016/0014-5793(79)80407-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masters S. B., Stroud R. M., Bourne H. R. Family of G protein alpha chains: amphipathic analysis and predicted structure of functional domains. Protein Eng. 1986 Oct-Nov;1(1):47–54. [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Rubin J. R., Morikawa K., Nyborg J., la Cour T. F., Clark B. F., Miller D. L. Structural features of the GDP binding site of elongation factor Tu from Escherichia coli as determined by x-ray diffraction. FEBS Lett. 1981 Jun 29;129(1):177–179. doi: 10.1016/0014-5793(81)80784-3. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Kamen R. I., Schleif R. F. Factor necessary for ribosomal RNA synthesis. Nature. 1970 Nov 21;228(5273):748–751. doi: 10.1038/228748a0. [DOI] [PubMed] [Google Scholar]
- Van Noort J. M., Kraal B., Sinjorgo K. M., Persoon N. L., Johanns E. S., Bosch L. Methylation in vivo of elongation factor EF-Tu at lysine-56 decreases the rate of tRNA-dependent GTP hydrolysis. Eur J Biochem. 1986 Nov 3;160(3):557–561. doi: 10.1111/j.1432-1033.1986.tb10074.x. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Neidhardt F. C. Ribosomes as sensors of heat and cold shock in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5589–5593. doi: 10.1073/pnas.87.15.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. C., Alvarez J. D., Bernlohr R. W. Nutrient-dependent methylation of a membrane-associated protein of Escherichia coli. J Bacteriol. 1990 Sep;172(9):5147–5153. doi: 10.1128/jb.172.9.5147-5153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]