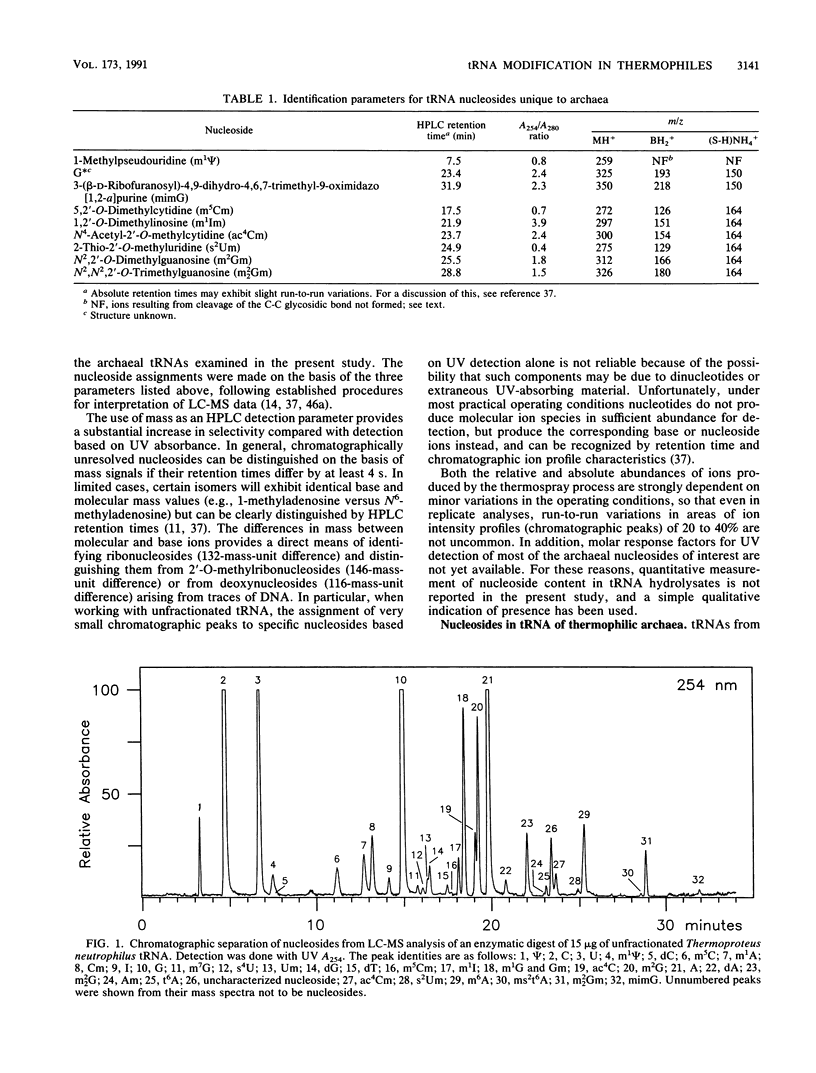
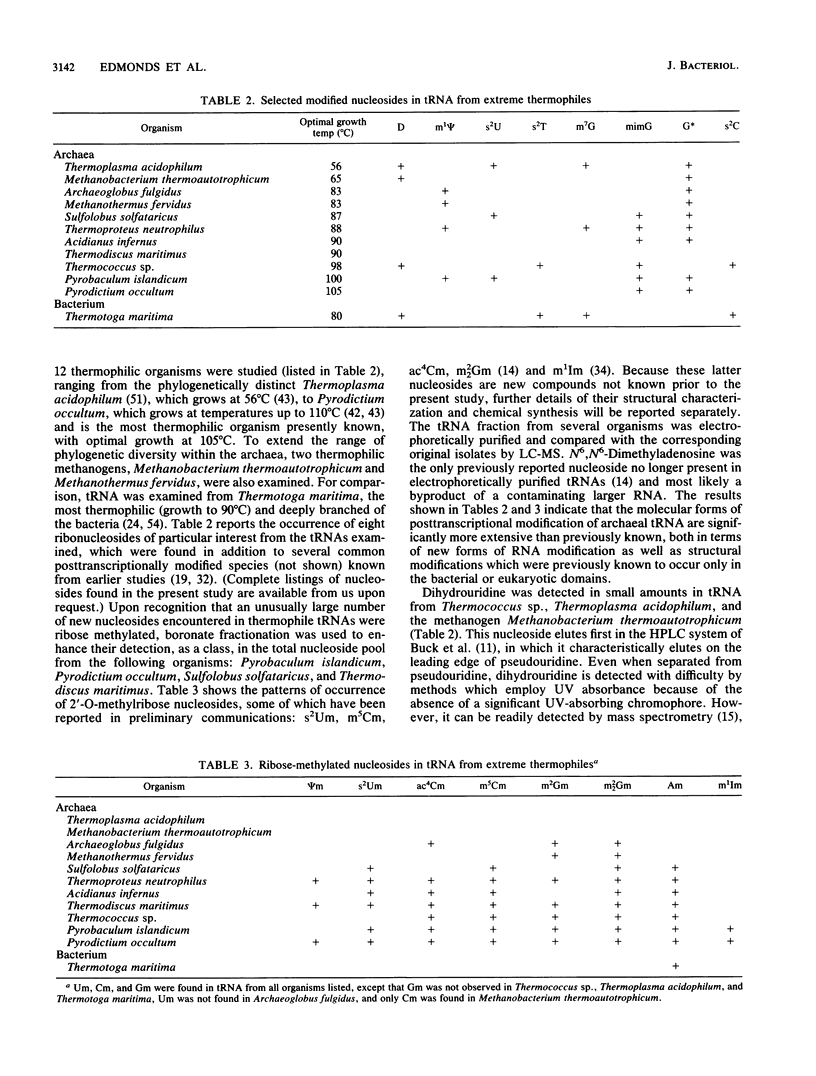
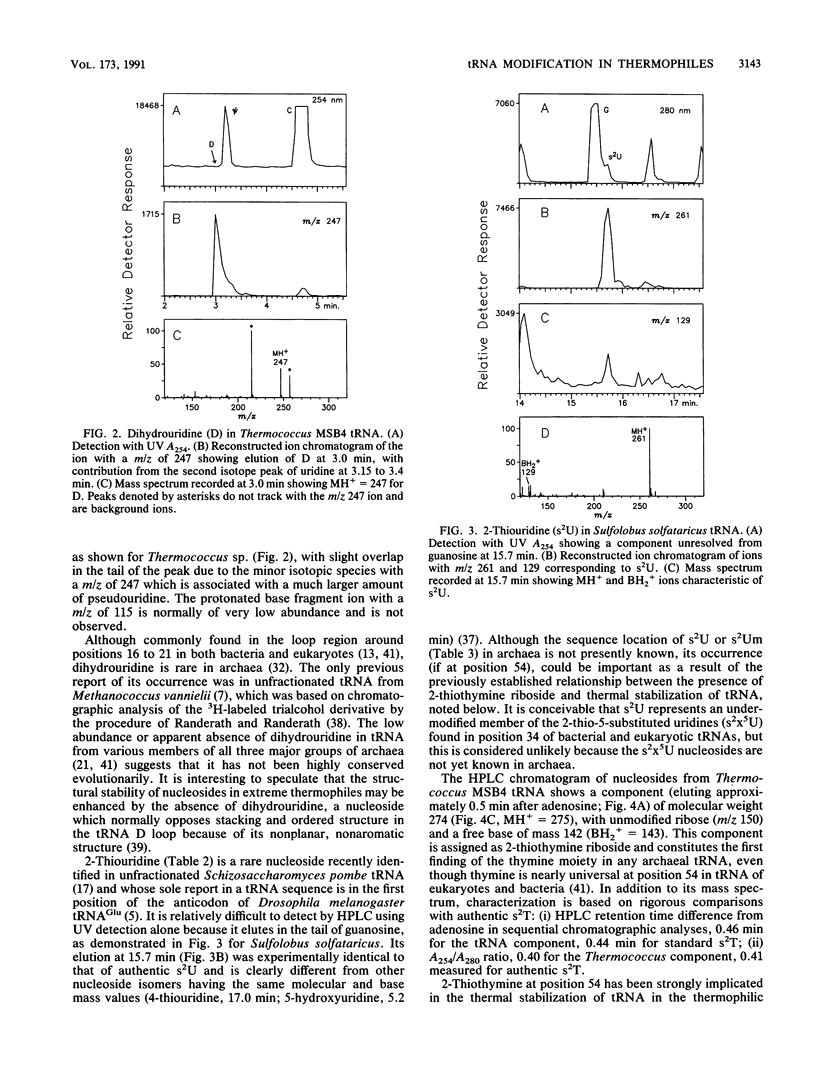
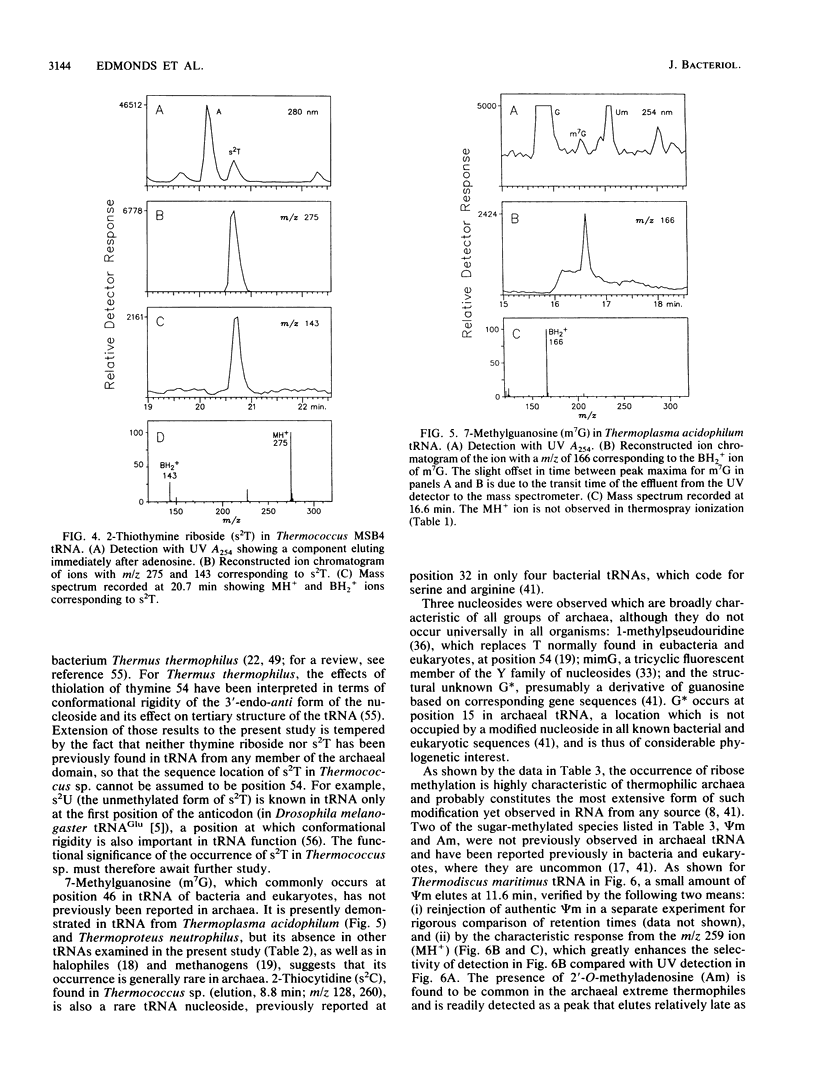
Abstract
Nucleoside modification has been studied in unfractionated tRNA from 11 thermophilic archaea (archaebacteria), including phylogenetically diverse representatives of thermophilic methanogens and sulfur-metabolizing hyperthermophiles which grow optimally in the temperature range of 56 (Thermoplasma acidophilum) to 105 degrees C (Pyrodictium occultum), and for comparison from the most thermophilic bacterium (eubacterium) known, Thermotoga maritima (80 degrees C). Nine nucleosides are found to be unique to the archaea, six of which are structurally novel in being modified both in the base and by methylation in ribose and occur primarily in tRNA from the extreme thermophiles in the Crenarchaeota of the archaeal phylogenetic tree. 2-Thiothymine occurs in tRNA from Thermococcus sp., and constitutes the only known occurrence of the thymine moiety in archaeal RNA, in contrast to its near-ubiquitous presence in tRNA from bacteria and eukarya. A total of 33 modified nucleosides are rigorously characterized in archaeal tRNA in the present study, demonstrating that the structural range of posttranscriptional modifications in archaeal tRNA is more extensive than previously known. From a phylogenetic standpoint, certain tRNA modifications occur in the archaea which are otherwise unique to either the bacterial or eukaryal domain, although the overall patterns of modification are more typical of eukaryotes than bacteria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1959;32(3):270–277. doi: 10.1007/BF00409348. [DOI] [PubMed] [Google Scholar]
- Achenbach-Richter L., Gupta R., Stetter K. O., Woese C. R. Were the original eubacteria thermophiles? Syst Appl Microbiol. 1987;9:34–39. doi: 10.1016/s0723-2020(87)80053-x. [DOI] [PubMed] [Google Scholar]
- Achenbach-Richter L., Stetter K. O., Woese C. R. A possible biochemical missing link among archaebacteria. Nature. 1987 May 28;327(6120):348–349. doi: 10.1038/327348a0. [DOI] [PubMed] [Google Scholar]
- Agris P. F., Koh H., Söll D. The effect of growth temperatures on the in vivo ribose methylation of Bacillus stearothermophilus transfer RNA. Arch Biochem Biophys. 1973 Jan;154(1):277–282. doi: 10.1016/0003-9861(73)90058-1. [DOI] [PubMed] [Google Scholar]
- Altwegg M., Kubli E. The nucleotide sequence of glutamate tRNA4 of Drosophila melanogaster. Nucleic Acids Res. 1980 Jan 25;8(2):215–223. doi: 10.1093/nar/8.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Best A. N. Composition and Characterization of tRNA from Methanococcus vannielii. J Bacteriol. 1978 Jan;133(1):240–250. doi: 10.1128/jb.133.1.240-250.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Ericson J. U., Gustafsson C. E., Hagervall T. G., Jönsson Y. H., Wikström P. M. Transfer RNA modification. Annu Rev Biochem. 1987;56:263–287. doi: 10.1146/annurev.bi.56.070187.001403. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Buck M., Connick M., Ames B. N. Complete analysis of tRNA-modified nucleosides by high-performance liquid chromatography: the 29 modified nucleosides of Salmonella typhimurium and Escherichia coli tRNA. Anal Biochem. 1983 Feb 15;129(1):1–13. doi: 10.1016/0003-2697(83)90044-1. [DOI] [PubMed] [Google Scholar]
- Crain P. F. Preparation and enzymatic hydrolysis of DNA and RNA for mass spectrometry. Methods Enzymol. 1990;193:782–790. doi: 10.1016/0076-6879(90)93450-y. [DOI] [PubMed] [Google Scholar]
- Dirheimer G. Chemical nature, properties, location, and physiological and pathological variations of modified nucleosides in tRNAs. Recent Results Cancer Res. 1983;84:15–46. doi: 10.1007/978-3-642-81947-6_2. [DOI] [PubMed] [Google Scholar]
- Edmonds C. G., Vestal M. L., McCloskey J. A. Thermospray liquid chromatography-mass spectrometry of nucleosides and of enzymatic hydrolysates of nucleic acids. Nucleic Acids Res. 1985 Nov 25;13(22):8197–8206. doi: 10.1093/nar/13.22.8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Grossenbacher A. M., Stadelmann B., Heyer W. D., Thuriaux P., Kohli J., Smith C., Agris P. F., Kuo K. C., Gehrke C. Antisuppressor mutations and sulfur-carrying nucleosides in transfer RNAs of Schizosaccharomyces pombe. J Biol Chem. 1986 Dec 15;261(35):16351–16355. [PubMed] [Google Scholar]
- Gupta R. Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. J Biol Chem. 1984 Aug 10;259(15):9461–9471. [PubMed] [Google Scholar]
- Horie N., Hara-Yokoyama M., Yokoyama S., Watanabe K., Kuchino Y., Nishimura S., Miyazawa T. Two tRNAIle1 species from an extreme thermophile, Thermus thermophilus HB8: effect of 2-thiolation of ribothymidine on the thermostability of tRNA. Biochemistry. 1985 Oct 8;24(21):5711–5715. doi: 10.1021/bi00342a004. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of the tRNAMMet from the archaebacterium Thermoplasma acidophilum. Nucleic Acids Res. 1981 Sep 11;9(17):4387–4390. doi: 10.1093/nar/9.17.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Beier H., Akita N., Nishimura S. Natural UAG suppressor glutamine tRNA is elevated in mouse cells infected with Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1987 May;84(9):2668–2672. doi: 10.1073/pnas.84.9.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu N., Nishimura S. Analysis of modified nucleosides and nucleotide sequence of tRNA. Methods Enzymol. 1987;155:379–396. doi: 10.1016/0076-6879(87)55026-1. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Ihara M., Yabusaki Y., Nishimura S. Initiator tRNAs from archaebacteria show common unique sequence characteristics. Nature. 1982 Aug 12;298(5875):684–685. doi: 10.1038/298684a0. [DOI] [PubMed] [Google Scholar]
- Kumagai I., Watanabe K., Oshima T. A thermostable tRNA (guanosine-2')-methyltransferase from Thermus thermophilus HB27 and the effect of ribose methylation on the conformational stability of tRNA. J Biol Chem. 1982 Jul 10;257(13):7388–7395. [PubMed] [Google Scholar]
- Lothrop C. D., Jr, Uziel M. Rapid group separations of nucleotides and related compounds of silica columns. Anal Biochem. 1980 Nov 15;109(1):160–166. doi: 10.1016/0003-2697(80)90025-1. [DOI] [PubMed] [Google Scholar]
- McCloskey J. A., Crain P. F., Edmonds C. G., Gupta R., Hashizume T., Phillipson D. W., Stetter K. O. Structure determination of a new fluorescent tricyclic nucleoside from archaebacterial tRNA. Nucleic Acids Res. 1987 Jan 26;15(2):683–693. doi: 10.1093/nar/15.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang H., Ihara M., Kuchino Y., Nishimura S., Gupta R., Woese C. R., McCloskey J. A. Structure of a modified nucleoside in archaebacterial tRNA which replaces ribosylthymine. 1-Methylpseudouridine. J Biol Chem. 1982 Apr 10;257(7):3589–3592. [PubMed] [Google Scholar]
- Pomerantz S. C., McCloskey J. A. Analysis of RNA hydrolyzates by liquid chromatography-mass spectrometry. Methods Enzymol. 1990;193:796–824. doi: 10.1016/0076-6879(90)93452-q. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetter K. O., Lauerer G., Thomm M., Neuner A. Isolation of extremely thermophilic sulfate reducers: evidence for a novel branch of archaebacteria. Science. 1987 May 15;236(4803):822–824. doi: 10.1126/science.236.4803.822. [DOI] [PubMed] [Google Scholar]
- Takeda N., Pomerantz S. C., McCloskey J. A. Detection of ribose-methylated nucleotides in enzymatic hydrolysates of RNA by thermospray liquid chromatography-mass spectrometry. J Chromatogr. 1991 Jan 2;562(1-2):225–235. doi: 10.1016/0378-4347(91)80580-6. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Saneyoshi M., Nishimura S. Replacement of ribothymidine by 5-methyl-2-thiouridine in sequence GT psi C in tRNA of an extreme thermophile. FEBS Lett. 1974 Jul 1;43(1):59–63. doi: 10.1016/0014-5793(74)81105-1. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Tsai L., Ching W. M., Stadtman T. C. Identification and synthesis of a naturally occurring selenonucleoside in bacterial tRNAs: 5-[(methylamino)methyl]-2-selenouridine. Biochemistry. 1984 Sep 25;23(20):4650–4655. doi: 10.1021/bi00315a021. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Gupta R. Are archaebacteria merely derived 'prokaryotes'? Nature. 1981 Jan 1;289(5793):95–96. doi: 10.1038/289095a0. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Olsen G. J. Archaebacterial phylogeny: perspectives on the urkingdoms. Syst Appl Microbiol. 1986;7:161–177. doi: 10.1016/s0723-2020(86)80001-7. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe K., Miyazawa T. Dynamic structures and functions of transfer ribonucleic acids from extreme thermophiles. Adv Biophys. 1987;23:115–147. doi: 10.1016/0065-227x(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Watanabe T., Murao K., Ishikura H., Yamaizumi Z., Nishimura S., Miyazawa T. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4905–4909. doi: 10.1073/pnas.82.15.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zillig W., Palm P., Reiter W. D., Gropp F., Pühler G., Klenk H. P. Comparative evaluation of gene expression in archaebacteria. Eur J Biochem. 1988 May 2;173(3):473–482. doi: 10.1111/j.1432-1033.1988.tb14023.x. [DOI] [PubMed] [Google Scholar]


