Abstract
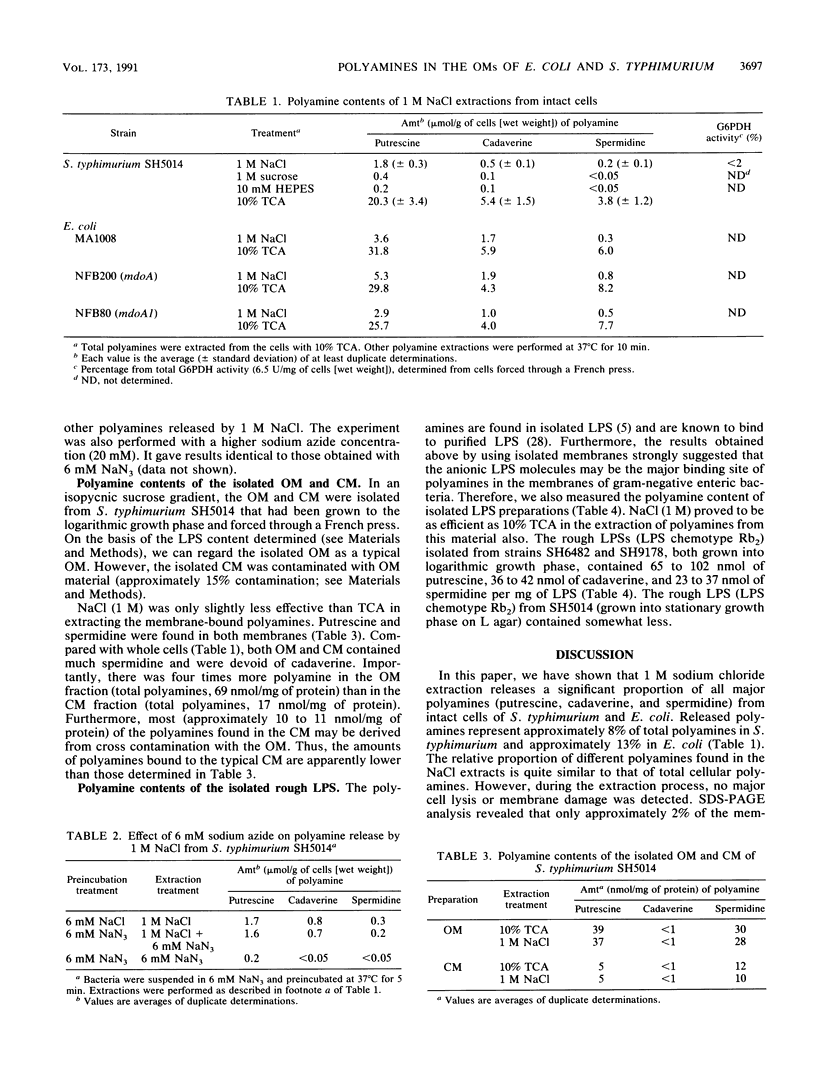
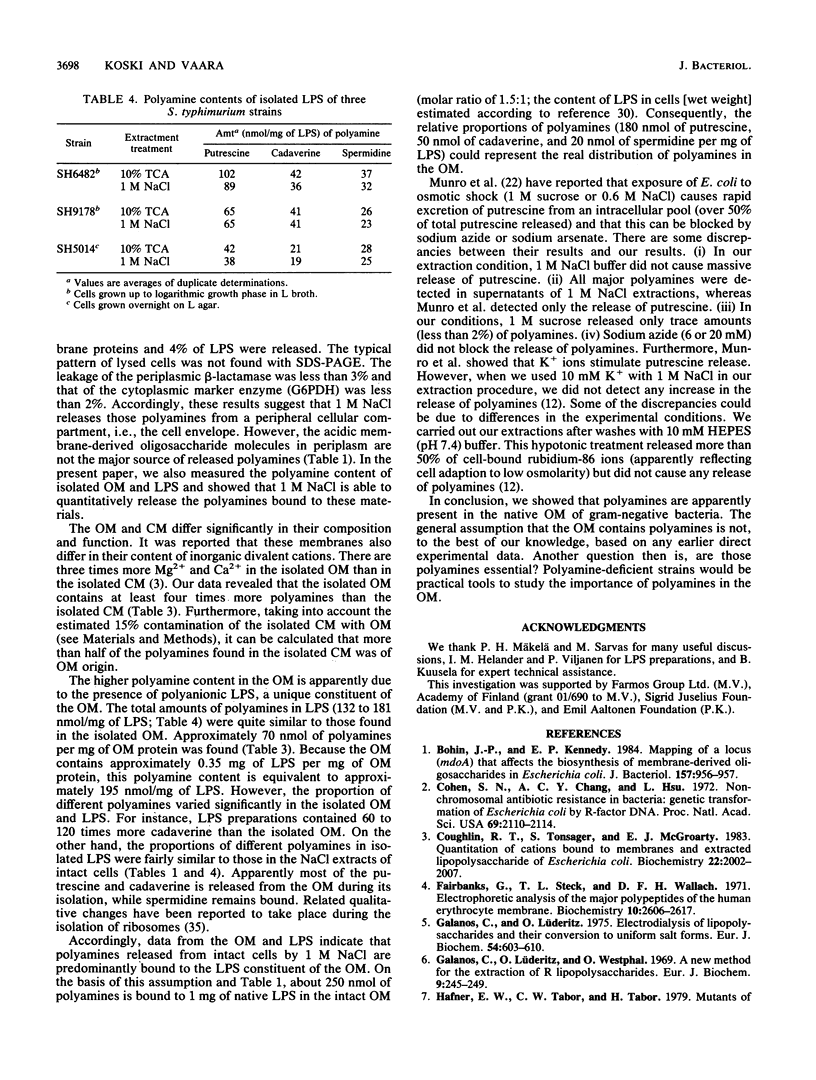
Extraction of whole cells of Salmonella typhimurium and Escherichia coli with 1 M NaCl released 8 to 13% of their total cellular polyamines (putrescine, cadaverine, and spermidine). This extraction did not cause significant cell lysis, release of outer membrane (OM) constituents, or leakage of periplasmic beta-lactamase. The extraction released nearly equal amounts of polyamines from mdo (membrane-derived oligosaccharide) mutants and wild type. These findings suggest that the released polyamines are apparently bound to the cell envelope. NaCl (1 M) was as effective as trichloroacetic acid in releasing polyamines from isolated OM and lipopolysaccharide (LPS). Isolated OM contained four times more polyamines than the cytoplasmic membrane. The increased binding to the OM is apparently due to the association of polyamines with the polyanionic LPS. Nearly identical amounts of polyamines were found in the OM and LPS preparations (as quantified per milligram of LPS). These amounts are equal to those released from the intact cells by 1 M NaCl (quantitation as above). However, redistribution of polyamines took place after cell disruption, because the relative proportions of different polyamines varied in the OM and LPS preparations. These results indicate that polyamines released from intact cells during 1 M NaCl extraction are preferentially derived from the OM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohin J. P., Kennedy E. P. Mapping of a locus (mdoA) that affects the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. J Bacteriol. 1984 Mar;157(3):956–957. doi: 10.1128/jb.157.3.956-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin R. T., Tonsager S., McGroarty E. J. Quantitation of metal cations bound to membranes and extracted lipopolysaccharide of Escherichia coli. Biochemistry. 1983 Apr 12;22(8):2002–2007. doi: 10.1021/bi00277a041. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Mutants of Escherichia coli that do not contain 1,4-diaminobutane (putrescine) or spermidine. J Biol Chem. 1979 Dec 25;254(24):12419–12426. [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Helander I. M., Vaara M., Sukupolvi S., Rhen M., Saarela S., Zähringer U., Mäkelä P. H. rfaP mutants of Salmonella typhimurium. Eur J Biochem. 1989 Nov 20;185(3):541–546. doi: 10.1111/j.1432-1033.1989.tb15147.x. [DOI] [PubMed] [Google Scholar]
- Homma T., Nakae T. Effects of cations on the outer membrane permeability of Escherichia coli. Tokai J Exp Clin Med. 1982;7 (Suppl):171–175. [PubMed] [Google Scholar]
- Hukari R., Helander I. M., Vaara M. Chain length heterogeneity of lipopolysaccharide released from Salmonella typhimurium by ethylenediaminetetraacetic acid or polycations. Eur J Biochem. 1986 Feb 3;154(3):673–676. doi: 10.1111/j.1432-1033.1986.tb09450.x. [DOI] [PubMed] [Google Scholar]
- Koski P., Helander I. M., Sarvas M., Vaara M. Analysis of polyamines as their dabsyl derivatives by reversed-phase high-performance liquid chromatography. Anal Biochem. 1987 Jul;164(1):261–266. doi: 10.1016/0003-2697(87)90395-2. [DOI] [PubMed] [Google Scholar]
- Koski P., Rhen M., Kantele J., Vaara M. Isolation, cloning, and primary structure of a cationic 16-kDa outer membrane protein of Salmonella typhimurium. J Biol Chem. 1989 Nov 15;264(32):18973–18980. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- MAGER J. The stabilizing effect of spermine and related polyamines and bacterial protoplasts. Biochim Biophys Acta. 1959 Dec;36:529–531. doi: 10.1016/0006-3002(59)90195-7. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Morris D. R., Koffron K. L. Putrescine biosynthesis in Escherichia coli. Regulation through pathway selection. J Biol Chem. 1969 Nov 25;244(22):6094–6099. [PubMed] [Google Scholar]
- Munro G. F., Hercules K., Morgan J., Sauerbier W. Dependence of the putrescine content of Escherichia coli on the osmotic strength of the medium. J Biol Chem. 1972 Feb 25;247(4):1272–1280. [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Song S. A., Shaltiel L., Nurminen M. Outer membrane of Salmonella XIV. Reduced transmembrane diffusion rates in porin-deficient mutants. Biochem Biophys Res Commun. 1976 May 23;76(2):324–330. doi: 10.1016/0006-291x(77)90728-8. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson A. A., Hancock R. E., McGroarty E. J. Binding of polycationic antibiotics and polyamines to lipopolysaccharides of Pseudomonas aeruginosa. J Bacteriol. 1985 Dec;164(3):1256–1261. doi: 10.1128/jb.164.3.1256-1261.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T. T., Cohen S. S. Effects of polyamines on the structure and reactivity of tRNA. Prog Nucleic Acid Res Mol Biol. 1976;17:15–42. doi: 10.1016/s0079-6603(08)60064-1. [DOI] [PubMed] [Google Scholar]
- Smit J., Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: chemical analysis and freeze-fracture studies with lipopolysaccharide mutants. J Bacteriol. 1975 Nov;124(2):942–958. doi: 10.1128/jb.124.2.942-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souzu H. Fluorescence polarization studies on Escherichia coli membrane stability and its relation to the resistance of the cell to freeze-thawing. II. Stabilization of the membranes by polyamines. Biochim Biophys Acta. 1986 Oct 9;861(2):361–367. doi: 10.1016/0005-2736(86)90439-6. [DOI] [PubMed] [Google Scholar]
- Stocker B. A., Nurminen M., Mäkelä P. H. Mutants defective in the 33K outer membrane protein of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):376–383. doi: 10.1128/jb.139.2.376-383.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Dobbs L. G. Metabolism of 1,4-diaminobutane and spermidine in Escherichia coli: the effects of low temperature during storage and harvesting of cultures. J Biol Chem. 1970 Apr 25;245(8):2086–2091. [PubMed] [Google Scholar]
- Tabor C. W., Kellogg P. D. The effect of isolation conditions on the polyamine content of Escherichia coli ribosomes. J Biol Chem. 1967 Mar 10;242(5):1044–1052. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines in microorganisms. Microbiol Rev. 1985 Mar;49(1):81–99. doi: 10.1128/mr.49.1.81-99.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Quantitative determination of naturally occurring aliphatic diamines and polyamines by an automated liquid chromatography procedure. Methods Enzymol. 1983;94:29–36. doi: 10.1016/s0076-6879(83)94006-5. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations sensitize enteric bacteria to antibiotics. Antimicrob Agents Chemother. 1983 Jul;24(1):107–113. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]


