Abstract
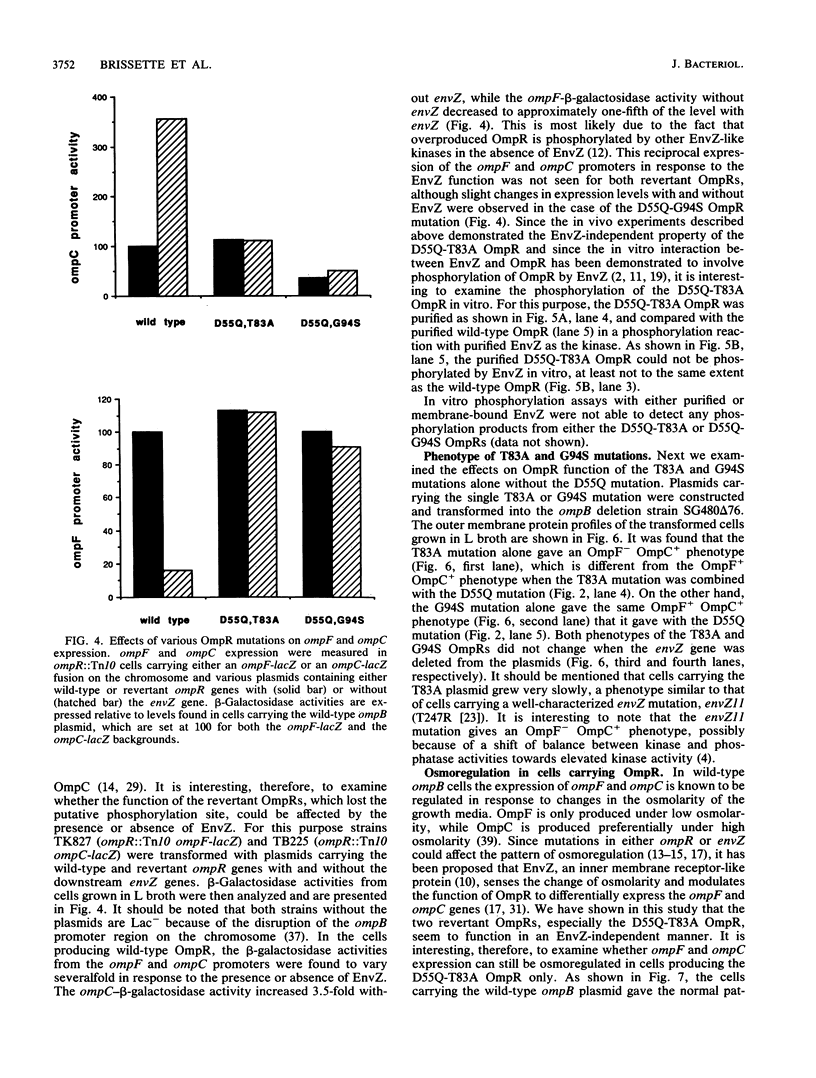
OmpR is a transcriptional activator for the ompF and ompC genes of Escherichia coli. Its phosphorylation is mediated by a transmembrane sensory-receptor protein, EnvZ, and is essential for transcriptional activation. In a previous study, when the aspartic acid residue at position 55, the putative phosphorylation site, was replaced with glutamine (D55Q), ompF and ompC expression were completely lost. In this study two pseudorevertants of the D55Q mutation were isolated and identified to be the replacement of threonine at position 83 with alanine (T83A) and glycine at position 94 with serine (G94S). The revertant OmpRs no longer responded to EnvZ function when ompF and ompC expression were examined. The purified D55Q-T83A OmpR was unable to be phosphorylated by EnvZ in vitro. The role of EnvZ as an osmosensor for the environmentally regulated expression of OmpF and OmpC has been indicated in previous studies. The isolation of seemingly EnvZ-independent OmpR revertants in this study, however, made it possible to examine the osmolarity-regulated expression of OmpF and OmpC in the absence of effects exerted by EnvZ. We found that the expression of OmpF and OmpC supported by these revertant OmpRs was clearly regulated in accordance with the change in osmolarity of the growth media. These results indicate that another EnvZ-independent mechanism(s) may also contribute to the regulated expression of the ompF and ompC genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Mizuno T., Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1989 May 25;264(15):8563–8567. [PubMed] [Google Scholar]
- Aiba H., Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, stimulates the transcription of the ompF and ompC genes in Escherichia coli. FEBS Lett. 1990 Feb 12;261(1):19–22. doi: 10.1016/0014-5793(90)80626-t. [DOI] [PubMed] [Google Scholar]
- Aiba H., Nakasai F., Mizushima S., Mizuno T. Evidence for the physiological importance of the phosphotransfer between the two regulatory components, EnvZ and OmpR, in osmoregulation in Escherichia coli. J Biol Chem. 1989 Aug 25;264(24):14090–14094. [PubMed] [Google Scholar]
- Aiba H., Nakasai F., Mizushima S., Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, results in stimulation of its DNA-binding ability. J Biochem. 1989 Jul;106(1):5–7. doi: 10.1093/oxfordjournals.jbchem.a122817. [DOI] [PubMed] [Google Scholar]
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourret R. B., Hess J. F., Simon M. I. Conserved aspartate residues and phosphorylation in signal transduction by the chemotaxis protein CheY. Proc Natl Acad Sci U S A. 1990 Jan;87(1):41–45. doi: 10.1073/pnas.87.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette R. E., Tsung K. L., Inouye M. Suppression of a mutation in OmpR at the putative phosphorylation center by a mutant EnvZ protein in Escherichia coli. J Bacteriol. 1991 Jan;173(2):601–608. doi: 10.1128/jb.173.2.601-608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau D. E., Ikenaka K., Tsung K. L., Inouye M. Primary characterization of the protein products of the Escherichia coli ompB locus: structure and regulation of synthesis of the OmpR and EnvZ proteins. J Bacteriol. 1985 Nov;164(2):578–584. doi: 10.1128/jb.164.2.578-584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Comeau D., Norioka S., Inouye M. Localization and membrane topology of EnvZ, a protein involved in osmoregulation of OmpF and OmpC in Escherichia coli. J Biol Chem. 1987 Dec 5;262(34):16433–16438. [PubMed] [Google Scholar]
- Forst S., Delgado J., Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Rampersaud A., Inouye M. In vivo phosphorylation of OmpR, the transcription activator of the ompF and ompC genes in Escherichia coli. J Bacteriol. 1990 Jun;172(6):3473–3477. doi: 10.1128/jb.172.6.3473-3477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Inouye M. Environmentally regulated gene expression for membrane proteins in Escherichia coli. Annu Rev Cell Biol. 1988;4:21–42. doi: 10.1146/annurev.cb.04.110188.000321. [DOI] [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J., Berman M. L. Isolation and characterization of delta ompB strains of Escherichia coli by a general method based on gene fusions. J Bacteriol. 1985 May;162(2):840–844. doi: 10.1128/jb.162.2.840-844.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett S., Taylor R. K., Silhavy T. J. Isolation and characterization of chain-terminating nonsense mutations in a porin regulator gene, envZ. J Bacteriol. 1983 Oct;156(1):62–69. doi: 10.1128/jb.156.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeme-Cook K. A., May G., Bremer E., Higgins C. F. Osmotic regulation of porin expression: a role for DNA supercoiling. Mol Microbiol. 1989 Sep;3(9):1287–1294. doi: 10.1111/j.1365-2958.1989.tb00279.x. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Huang L., Tsui P., Freundlich M. Integration host factor is a negative effector of in vivo and in vitro expression of ompC in Escherichia coli. J Bacteriol. 1990 Sep;172(9):5293–5298. doi: 10.1128/jb.172.9.5293-5298.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Silhavy T. J. A bacterial environmental sensor that functions as a protein kinase and stimulates transcriptional activation. Genes Dev. 1989 May;3(5):598–605. doi: 10.1101/gad.3.5.598. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Jo Y. L., Nara F., Ichihara S., Mizuno T., Mizushima S. Purification and characterization of the OmpR protein, a positive regulator involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1986 Nov 15;261(32):15252–15256. [PubMed] [Google Scholar]
- Matsuyama S., Mizuno T., Mizushima S. Interaction between two regulatory proteins in osmoregulatory expression of ompF and ompC genes in Escherichia coli: a novel ompR mutation suppresses pleiotropic defects caused by an envZ mutation. J Bacteriol. 1986 Dec;168(3):1309–1314. doi: 10.1128/jb.168.3.1309-1314.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Wurtzel E. T., Inouye M. Cloning of the regulatory genes (ompR and envZ) for the matrix proteins of the Escherichia coli outer membrane. J Bacteriol. 1982 Jun;150(3):1462–1466. doi: 10.1128/jb.150.3.1462-1466.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara F., Matsuyama S., Mizuno T., Mizushima S. Molecular analysis of mutant ompR genes exhibiting different phenotypes as to osmoregulation of the ompF and ompC genes of Escherichia coli. Mol Gen Genet. 1986 Feb;202(2):194–199. doi: 10.1007/BF00331636. [DOI] [PubMed] [Google Scholar]
- Norioka S., Ramakrishnan G., Ikenaka K., Inouye M. Interaction of a transcriptional activator, OmpR, with reciprocally osmoregulated genes, ompF and ompC, of Escherichia coli. J Biol Chem. 1986 Dec 25;261(36):17113–17119. [PubMed] [Google Scholar]
- Sanders D. A., Gillece-Castro B. L., Stock A. M., Burlingame A. L., Koshland D. E., Jr Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J Biol Chem. 1989 Dec 25;264(36):21770–21778. [PubMed] [Google Scholar]
- Slauch J. M., Garrett S., Jackson D. E., Silhavy T. J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock A. M., Mottonen J. M., Stock J. B., Schutt C. E. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature. 1989 Feb 23;337(6209):745–749. doi: 10.1038/337745a0. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Stock A. M., Mottonen J. M. Signal transduction in bacteria. Nature. 1990 Mar 29;344(6265):395–400. doi: 10.1038/344395a0. [DOI] [PubMed] [Google Scholar]
- Tsui P., Helu V., Freundlich M. Altered osmoregulation of ompF in integration host factor mutants of Escherichia coli. J Bacteriol. 1988 Oct;170(10):4950–4953. doi: 10.1128/jb.170.10.4950-4953.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung K., Brissette R. E., Inouye M. Enhancement of RNA polymerase binding to promoters by a transcriptional activator, OmpR, in Escherichia coli: its positive and negative effects on transcription. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5940–5944. doi: 10.1073/pnas.87.15.5940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsung K., Brissette R. E., Inouye M. Identification of the DNA-binding domain of the OmpR protein required for transcriptional activation of the ompF and ompC genes of Escherichia coli by in vivo DNA footprinting. J Biol Chem. 1989 Jun 15;264(17):10104–10109. [PubMed] [Google Scholar]
- Utsumi R., Brissette R. E., Rampersaud A., Forst S. A., Oosawa K., Inouye M. Activation of bacterial porin gene expression by a chimeric signal transducer in response to aspartate. Science. 1989 Sep 15;245(4923):1246–1249. doi: 10.1126/science.2476847. [DOI] [PubMed] [Google Scholar]







